Office of Research & Development |
 |

The revised Project Cover Sheet (PCS) wizard is available in the IRBNet sandbox the week of December 11. The updated wizard outline (VA network access only) is posted on the VAIRRS SharePoint portal under the ORPP&E Standard Form Library menu option. The VA Project Lead/Principal Investigator and Additional Project Personnel sections moved to the Study Team Tracking wizard. The IRB of Record Type will appear for all studies that select "Data and/or biospecimens from living human individuals." On the PCS PDF both the "Funding Source Code" and the "Funding Source Code - Other" response will now appear.
Study teams will not be required to update previous PCS submissions unless there has been a change to the study.

The auto-generated notifications can be customized for your site. Please contact IRBNet Support at govsupport@irbnet.org for more information.

Recusals can be noted on the Reviews and Minutes page. Under the Voting section, you can add or remove recused committee members, and the recused members will be recorded in the minutes.

At the end of 2023, the VAIRRS Support Team incorporated a standard numbering catalogue system into the existing naming convention used for all forms within IRBNet and SharePoint. Most forms in the library already used the numbering catalogue system, but it has now been implemented across the board to improve user navigation within the library.
During the first half of the new year, the VAIRRS Support Team will be performing a retrospective functional audit of all documents that comprise the Standard Form Library within the VAIRRS SharePoint and IRBNet. The purpose of this audit is to assess aspects of document functionality and make updates as needed to ensure users have access to high-quality, standardized forms and templates. This will include migrating existing forms and templates into a new document format.

The VAIRRS Strategic Advisory Council (VSAC) recently met to review current personnel tracking processes. The VSAC determined personnel tracking should occur separately from project coversheet tracking. This will provide a more efficient means of updating study personnel and associated training requirements. The VAIRRS team is working on a draft personnel tracking form. We will provide further updates as development progresses.

The data entered into the RCO audit module, released in Spring 2023, is ready for export. RCOs who would like an aggregate extract of the FDC data must contact the IRBNet Help Desk at govsupport@irbnet.org. As a reminder, the RCO must have entered audit results into the audit module to receive an extract.

Reminder: New Project Submission Forms were the latest forms to be revised and were released on October 25, 2023. All New Project Submission Forms must be used as of January 2, 2024. Prior to using these new forms, please read the Summary of Changes – CIRB New Project Forms for a detailed list of forms and process changes. For additional information regarding these changes, you may also refer to the VAIRRS webinar from October 24, 2023, titled “CIRB Forms and Process Updates.”

VAIRRS has added a new Project Closure and Withdrawal User Guide (VA network access only) for the purpose of walking administrators/submission coordinators through the process of performing a researcher-initiated closure, performing an administrative closure, and withdrawing an erroneous package. Users can access the new guide within VAIRRS University either by selecting the "Administrator/Submission Coordinator" user role or by navigating to the "VAIRRS User Guides" training material category.
To learn more about VAIRRS University, view the VAIRRS University walk-through webinar, led by VAIRRS experts.
For questions about VAIRRS University, contact the VAIRRS Support Team at VAIRRS@va.gov.

Click below to watch tutorials on how to create and submit COI disclosures within VAIRRS:


The VAIRRS Mentor Program currently has six team members from various roles across the VA providing mentorship, with a backlog of mentees awaiting the availability of a mentor.
We currently have 10 mentor vacancies. Please let us know if you would like to nominate a potential VAIRRS Mentor. Applications are accepted on an ongoing basis until all mentor slots are filled.
If you are interested in helping fellow VAIRRS users, you can access the mentor application via the VAIRRS Mentor Program SharePoint page (VA network access only).

The next expired email notification will be sent on June 15, 2024. The expired projects notification is sent to contacts directly from the Research Office Contact Verification (VA network access only) SharePoint list. Only primary and secondary site liaisons have permission to access this SharePoint list to make updates. If there is a change in a role in the list, contact your facility's primary or secondary site liaison to update the list.
If your site's site liaison has transitioned, please contact the VAIRRS Support Team Inbox VAIRRS@va.gov for assistance.

When assigning a package for ISSO (information system security officer) or PO (privacy officer) review in the research administration or R&DC (Research & Development Committee) workspaces be sure to select the appropriate reviewer designation from the drop-down list. The ISSO and PO reviewer designations are very helpful when tracking reviewer activity.
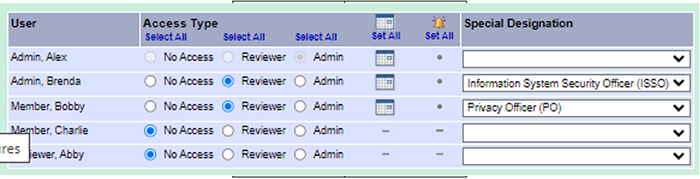

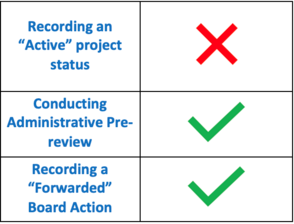 |
Did you miss this month’s VAIRRS webinar, “How to Properly Utilize the Research Admin Workspace in IRBNet”? If so, you are encouraged to review the webinar recording and available training resources. A summary of the activities discussed at the webinar are highlighted in the table to the left. |

FIND Pro Launched on June 13, 2023.
How FIND Pro empowers researchers:
Types of Documents included: All VA and VHA Directives, all VA and VHA Handbooks, ORD Program Guides–1200 Series, ORD Guidance and ORD FAQs.
Check It Out!
Office hours: Recurring office hours via Microsoft Teams started July 2023 and are held the second Tuesday and the fourth Wednesday each month. To attend the next sessions, select the links below:
Tuesday, September 12, 2023, 3:00-3:50 PM (EDT)
Wednesday, September 27, 2023, 12:00-12:50 PM (EDT)

Recent revisions to the combined Informed Consent Form (ICF)/HIPAA Authorization include the following that begin on page 8 and extend through page 10. This includes language clarifications from VHA Privacy.

During the February Change Control Board (CCB) meeting, the documents listed in the table below have been updated and added to the ORPP&E Standard Form Library within IRBNet and the VAIRRS SharePoint. The next VAIRRS CCB meeting is scheduled on March 21, 2024.
| Form/Letter/Wizard | Summary of Changes |
|---|---|
|
10.0W Project Cover Sheet Wizard |
The PI name was added, in addition to the name of the person/time of last edit, to the wizard PDF output. |
|
7.2A Combined ICF and HIPAA Template & 7.3A Informed Consent Template |
The word "template" was added in the front of the revision date in the footers within the 7.2A Combined ICF and HIPAA template and 7.3A Informed Consent Form template. |
|
3.6A – RDC Continuing Review Application or Final Closure (PDF) |
A text field was added to sub-item 7 under Section 2 – Project Status to allow users to provide the reason for closure. |
|
1IRB Information Sheet Wizard |
Various language changes were made within the wizard; however, the logic of the wizard was not impacted. To see the exact changes made, please refer to the latest version of the wizard outline linked here (VA network access only), or by navigating to the VAIRRS CCB Summary of Changes section within the VAIRRS SharePoint. |
|
Study Team Tracking Wizard |
Changes made to the wizard include the addition of an "N/A (Not Salaried by VA)" response option to the Outside Compensation question(s) and the addition of Investigator Details, as well as other minor changes. To see the exact changes made, please refer to the latest version of the wizard outline linked here (VA network access only), or by navigating to the VAIRRS CCB Summary of Changes section within the VAIRRS SharePoint. |
Reminder: The new Study Team Tracking (STT) wizard is available in the IRBNet as of December 11, 2023. The wizard outline is posted on the VAIRRS SharePoint portal under the ORPP&E Standard Form Library menu option. The personnel tracking data previously collected in the Project Cover Sheet (PCS) wizard will be collected in the STT wizard. The Outside Compensation and Financial Conflict of Interest responses will be collected in the STT wizard.
Study teams will be required to complete the new STT wizard for new submissions and to report changes in study team members.

Principal Investigator's (PI) concerned with sharing their or their team’s outside compensation with the rest of the team or the R&D committee can now use an alternative reporting solution, provided below.
Short-term workaround for existing projects:
Rationale:
The R&DC is not required to review outside salaries for existing projects, therefore the submission of information can take place outside of VAIRRS. The workaround meets the
requirement for posting the approval memo to the project file, and the only individuals with access to the salary information are the PI who submits, the ACOS, and the Authorizing Official. The R&DC members with access to the project will also have access to the internally published memo. If this is a concern, the Committee Administrator can remove all committee member access or just provide access to the Chair.

The Office of Research Reviews (ORR) is developing a series of information security instructions to support the research community. To kick off the series, ORR offers detailed instructions on an often-encountered issue delaying review of an Enterprise Research Data Security Plan (ERDSP) - providing full path names of electronic data storage locations.
The mapping of local drives is site specific. For example, the R: drive in Seattle is not the same as Cincinnati's R: drive. To complete an ERDSP for centralized information security review, ORR needs a drive's full path name, not the "R:" or "S:" but rather the more descriptive unique name starting with \\xxxx.va.gov. For anyone struggling with how to find that full path name, ORR has created an instructional document and short video.
Check out the instructions:
ERDSP Providing the Full Folder Path for Mapped Drives.pdf (VA network access only)
And the accompanying video:
ERDSP Providing the Full Folder Path for Mapped Drives Video Recording.mp4 (VA network access only)
As always, ORR is available to provide further clarification or assistance on all ERDSP-related topics. Feel free to let us know of any corrections or other areas of interest at OISISPSSDCDORRRESEARCHREVIEWTEAM@va.gov.