Office of Research & Development |
 |
Office of Research & Development |
 |
Prostate cancer occurs in the prostate, a walnut-sized gland that sits at the base of the bladder and is part of the male reproductive system. About one in eight men will be diagnosed with prostate cancer at some point in their lifetime. Prostate cancer is the most commonly diagnosed cancer among U.S. Veterans, making up 30% of new cancer diagnoses in the VA.
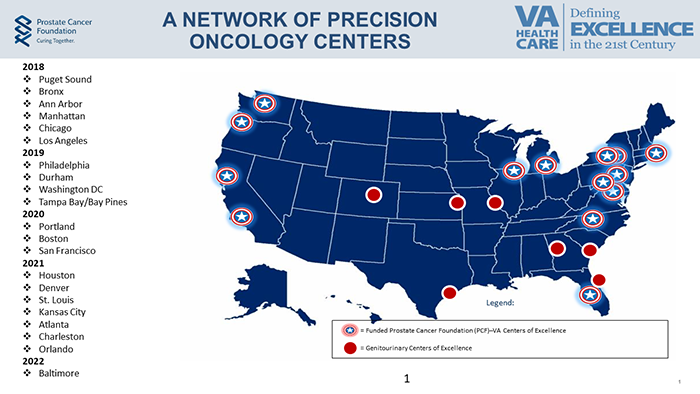
In 2016, VA Research partnered with the Prostate Cancer Foundation to establish the Precision Oncology Program for Cancer of the Prostate (POPCaP). The program uses genetic information to tailor individualized treatments for Veterans with advanced prostate cancer. Components of POPCaP include access to genetic testing and counseling, prostate cancer clinical trials, and FDA-approved drugs targeted to specific cancer mutations.
|
Precision Oncology Centers of Excellence |
Facility |
Principal Investigator |
|
The Stephen J. Cloobeck Precision Oncology Center of Excellence |
VA Puget Sound Health Care System Seattle, WA |
Bruce Montgomery |
|
The Michael and Lori Milken Family Foundation Precision Oncology Center of Excellence |
Greater Los Angeles VA Medical Center Los Angeles, CA |
Matthew Rettig |
|
The Robert Frederick Smith Precision Oncology Center of Excellence |
Jesse Brown VA Medical Center Chicago, IL |
Joshua Meeks |
|
The Stewart J. Rahr Foundation Precision Oncology Center of Excellence |
Ann Arbor VA Medical Center Ann Arbor, MI |
Phoebe Tsao |
|
The John and Daria Barry Foundation Precision Oncology Center of Excellence |
Manhattan VA Medical Center Manhattan, NY |
Danil Makarov |
|
The Blavatnik Family Foundation Precision Oncology Center of Excellence |
James J. Peters VA Medical Center Bronx, NY |
Antonio Fojo |
|
The Jonathan and Plum Simons Precision Oncology Center of Excellence |
Corporal Michael J. Crescenz VA Medical Center Philadelphia, PA |
Kyle Robinson Nevena Damjanov |
|
Prostate Cancer Foundation Precision Oncology Center of Excellence |
Durham VA Medical Center Durham, NC |
Rhonda Bitting |
|
The Edward P. Evans Foundation Precision Oncology Center of Excellence |
Washington VA Medical Center Washington, DC |
Manish Jain |
|
The John and Daria Barry Foundation Precision Oncology Center of Excellence |
James A. Haley Veterans Hospital Tampa, FL Bay Pines VA Medical Center Bay Pines, FL |
Kosj Yamoah |
|
Portland VA Medical Center Portland, OR |
Julie Graff |
|
|
Jamaica Plain VA Medical Center Boston, MA |
Chong-Xian Pan |
|
|
San Francisco VA Medical Center San Francisco, CA |
Franklin Huang |
|
|
Baltimore VA Medical Center Baltimore, MD |
Arif Hussain |
|
|
Atlanta VA Medical Center Atlanta, GA |
Wayne Harris Maria Ribeiro |
|
|
Ralph H. Johnson VA Medical Center Charleston, SC |
Steven Savage |
|
|
Rocky Mountain Regional VA Medical Center Denver, CO |
Daniel Bowles |
|
|
Michael E. DeBakey VA Medical Center Houston, TX |
Anita Sabichi |
|
|
Kansas City VA Medical Center Kansas City, MO |
Linda Verkruyse |
|
|
John J. Cochran Veterans Hospital St. Louis, MO |
Eric Knoche |
In support of the POPCaP program, VA launched the Prostate Cancer Analysis for Therapy Choice (PATCH) initiative. This effort has increased the number of VHA facilities involved in prostate cancer clinical trials and the number of Veterans participating in precision medicine studies. In addition, it has grown the number of physicians who provide care for prostate cancer patients and conduct prostate cancer research.
View Studies
|
Trial |
Details |
Status |
Locations |
|
High-dose Testosterone in Men with Metastatic Castration-resistant Prostate Cancer and ATM or CDK12 deficiency |
Recruiting |
Atlanta, Charleston, Connecticut, Denver, Durham, Gainesville, Houston, Kansas City, Louisville, Orlando, Portland, Puget Sound, St. Louis |
|
|
A Phase 3, Two-part, Randomized, Open-label, Adaptive Study Comparing BMS-986365 versus Investigator’s Choice of Therapy Comprising Either Docetaxel or Second Androgen Receptor Pathway Inhibitor (ARPI), in Participants with Metastatic Castration-resistant Prostate Cancer (mCRPC) |
Not Yet Recruiting |
Denver, Philadelphia, San Francisco |
|
|
A Phase II Clinical Trial to Evaluate the Efficacy and Safety of Fam-Trastuzumab Deruxtecan-Nxki (ENHERTU) in HER2-Positive Metastatic Castration-Resistant Prostate Adenocarcinoma |
Not Yet Recruiting |
Durham, George Washington University, Kansas City, Madison, Portland, Puget Sound, St. Louis, Washington, DC |
|
|
A single-arm, open-label, phase II study of Checkpoint inhibitors in men with progressive Metastatic castrate resistant Prostate cancer characterized by a mismatch repair deficiency or biallelic CDK12 inactivation |
Recruiting |
Ann Arbor, Bay Pines, Bronx, Chicago, Durham, Los Angeles, New York, Philadelphia, Portland, Puget Sound, Richmond, San Francisco, Washington DC |
|
|
An open-label, multicenter phase II study to compare the efficacy of carboplatin as first-line followed by second-line olaparib versus olaparib as first-line followed by second-line carboplatin in the treatment of patients with castration resistant prostate cancer containing homologous recombination deficiency |
Recruiting |
Ann Arbor, Atlanta, Aurora, CO, Bay Pines, Boise, Bronx, Chicago, Durham, Kansas City, Los Angeles, Madison, WI, Minneapolis, New York, Orlando, Philadelphia, Portland, Puget Sound, San Juan PR, Washington DC |
|
|
Study of INKmune in patients with mCRPC |
Not Yet Recruiting |
Los Angeles, Puget Sound |
|
|
Study of Pembrolizumab (MK-3475) combination therapies in metastatic Castration-Resistant Prostate Cancer (MK-3475-365/KEYNOTE-365) |
Recruiting |
Manhattan, Portland, San Francisco |
|
|
MK2400-001 |
A Phase 3, open-label study of Ifinatamab Deruxtecan versus Docetaxel in participants with metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Start Up |
Denver, Long Beach, Memphis, Portland, Seattle, Washington, DC, West Haven |
|
A Phase 3 randomized, open-label study of MK-5684 versus alternative Abiraterone Acetate or Enzalutamide in participants with metastatic Castration-Resistant Prostate Cancer (mCRPC) previously treated with Next-generation Hormonal Agent (NHA) and Taxane-based chemotherapy |
Recruiting |
Baltimore, Bronx, Charleston, Durham, Miami, Los Angeles, Portland, San Francisco, Tampa |
|
|
A Phase 3, randomized, open-label study of MK-5684 versus alternative Abiraterone Acetate or Enzalutamide in participants with metastatic Castration-resistant Prostate Cancer (mCRPC) and have progressed on or after prior treatment with one Next-generation Hormonal Agent (NHA) for Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) |
Recruiting |
Baltimore, Bronx, Charleston, Durham, Miami, Los Angeles, Portland, San Francisco, Tampa |
|
|
PCR3001 |
A Phase 3 randomized, double-blinded, placebo-controlled study of JNJ-78278343, a T cell redirecting agent targeting human Kallikrein 2 versus placebo for metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Site selection & feasibility |
TBD |
|
PCR3002 |
A Phase 3 randomized, open-label multinational study of metastasis-directed radiotherapy in combination with JNJ-78278343, a T-cell-redirecting agent targeting human Kallikrein 2 versus metastasis-directed radiotherapy for Oligometastatic Hormone-sensitive Prostate |
Site selection & feasibility |
TBD |
|
RESTORE |
A single-arm, open-label phase II trial of bipolar androgen therapy (BAT) in men with metastatic castration resistant prostate cancer (mCRPC)—A comparison of post-abiraterone (Abi) versus post-enzalutamide (Enza) patients |
Recruiting |
Ann Arbor, Cleveland, Greater Los Angeles, Hines, Indianapolis |
|
A single-arm phase II study of Neoadjuvant Intensified Androgen Deprivation (leuprolide and abiraterone acetate) in combination with AKT Inhibition (capivasertib) for high-risk localized prostate cancer with PTEN Loss |
Recruiting |
Bronx, Charleston, Portland, Puget Sound |
|
|
Copper Cu64 PSMA I&T injection in patients with histologically proven Metastatic Prostate Cancer |
Active, not recruiting |
Bronx, Greater Los Angeles, Hines, Portland, San Francisco, St. Louis |
|
|
A Phase 3, multicenter, open-label study to test the diagnostic performance of Copper Cu 64 PSMA I&T PET/CT in staging of men with newly diagnosed, unfavorable, intermediate-risk, high-risk or very high-risk Prostate Cancer electing to undergo Radical Prostatectomy with Pelvic Lymph Node Dissection |
Recruiting |
Bronx, Greater Los Angeles, Hines, Portland, San Francisco, St. Louis |
|
|
Veterans Affairs seamless phase II/III randomized trial of STAndard Systemic theRapy With or Without PET-directed Local Therapy for OligoRecurrenT Prostate Cancer (STARPORT) |
Recruiting |
Ann Arbor, Bay Pines, Boston, Cleveland, Durham, New Jersey, Hines, Houston, Indianapolis, Kansas City, Long Beach, Los Angeles, Milwaukee, Minneapolis, Richmond, St. Louis, Maryland, New York Harbor, Philadelphia |
|
|
Randomized phase II trial of targeted radiation with no castration for mCRPC |
Recruiting |
Los Angeles, Long Beach, Hines |
|
|
A phase II randomized study of YONSA® (Abiraterone Acetate), Enzalutamide or Apalutamide as first line therapy in Veterans with Castrate-sensitive Prostate Cancer |
Recruiting |
Ann Arbor, Houston, Los Angeles, Manhattan, Montrose, Philadelphia, Portland, Puget Sound, Salisbury, St. Louis, Tampa Bay |
Eighty-five percent of men with prostate cancer have disease contained within the prostate gland itself. However, 20% of Veterans who undergo local therapy (radiation or surgery) will see their cancer return and spread outside the prostate.
Scientists now understand that prostate cancer can spread beyond the prostate itself, yet not disperse widely throughout the body. Evidence suggests that adding radiation or surgery that targets areas of cancer spread to standard systemic treatment (SST) for prostate cancer may be an effective treatment.
The STARPORT clinical trial (STAndard Systemic TheRapy With or Without PET-directed Local Therapy for OligoRecurrenT Prostate Cancer) is examining the benefits of local cancer therapy combined with SST to treat prostate cancer with limited spread. Researchers want to find out if this combination improves disease control compared to SST alone in Veterans with recurrent prostate cancer.