Office of Research & Development |
 |
Where in IRBNet can sites manage financial conflict of interest (COI) reviews?
Sites can manage financial COI reviews on the VAIRRS SharePoint portal (VA network access only) and document reviews using the sample letter template, "COI Acknowledgment Sample."
Does IRBNet link to TMS and CITI?
Yes, IRBNet supports the integration of TMS and CITI, so that records can be automatically created for users who link their IRBNet accounts to TMS or CITI. CITI integration is complete. TMS integration is in progress and is expected to be completed by early 2023.
Will IRBNet update the station’s ePROMISE records?
Currently, the system does not integrate with ePROMISE. The plan is to integrate VAIRRS with other ORD enterprise systems in the near future.
Can you tell us more about the impetus for the Feasibility Alignment and Scientific Review (FA+SR) checklist?
Users requested that the Feasibility and Alignment assessment be separate from the Scientific assessment, since many projects come with a scientific review. The form was created because so many facilities were not assessing feasibility until the end of the study, if at all. After thousands of dollars of review time conducted by committees, R&D committees were then challenging if the study could be done or altering the study, causing amendments to be filed with other committees, using even more committee time. Ultimately, the perceived non-completion rate, especially investigator-initiated studies, was the impetus. The burden on the research staff and committees to review projects that are not feasible or scientifically invalid is significant and takes time away from viable and scientifically valid studies.
If the project does not meet the definition of research, does the FA or SR evaluation stop?
Unless a researcher’s facility requires their review, the research service is not responsible for projects that do not meet the research definition. The quality office is responsible for projects that involve quality improvement/assurance or evidence-based practice, etc.
Can the FA+SR reviewer checklists be completed after submission to sub-committees, or is the expectation that R&D provides this review first and then repeats the evaluation after approval from the other committee?
The review needs to be completed by someone (not necessarily an R&D member, though they likely have the best grasp of the projects happening across the facility) who can assess whether the project can be accomplished in the proposed time frame by the provider submitting it and if it will interfere with other studies happening at the facility. A statement from the investigator’s supervisor stating they have the time and are qualified to conduct the project would go a long way in helping the person reviewing the form. The review is intended to be complete before it is assigned to any committee for review to reduce the burden on the committees of studies that are not feasible or scientifically valid.
Is it acceptable if a site considers feasibility and scientific merit, but lacks a formalized Feasibility Alignment and Scientific Review (FA+SR) process?
The use of the FA+SR checklists are encouraged, but not required if the facility has an established process in place. This process was established to help sites that do not have an existing FA+SR process. FA+SR is designed to determine whether the project has the appropriate resources and expertise to complete the study, whether the nature of the research is appropriate to be conducted at a given institution, and whether the project is sufficiently meritorious to proceed. The goal of FA+SR is to determine these factors at the beginning of the regulatory review process.
Is the burden of FA+SR shifted from the IRB to the R&DC? The workload of the R&DC has increased substantially and seems unsustainable.
FA+SR is an institutional responsibility and does not necessarily fall within the IRB’s purview. The goal of FA+SR is not to increase the workload of the R&DC. The VA Electronic Determination Aid (VAEDA) is now available to all VA facilities. VAEDA is intended to relieve the R&DC and IRB from reviewing projects that are not research, thereby reducing the burden on both committees. The initial FA review can be conducted by an R&DC member and is intended to keep projects that are not feasible from ever reaching the R&DC. Like the Enterprise Research Data Security Plan (ERDSP), if proposed technology has little to no chance of being acquired or approved within the VA environment, then hopefully, the R&DC will not have to review the project. The goal of FA+SR is to determine these factors at the beginning of the regulatory review process.
Will smart forms, fillable forms, wizards and letter templates be made available to the sites transitioning to VA’s instance of IRBNet (VAIRRS)?
ORPP&E, along with a field-based workgroup, has developed a core library of form and letter templates for each of the committee and subcommittee workspaces in VAIRRS. Users may preview the ORPP&E Standard Form Library (VA network access only) located within the VAIRRS SharePoint portal. The Project Cover Sheet wizard is required for all submissions. The IRB Information Sheet wizard is required for all submissions to the IRB (internal and external). A continuing review form, closure report and IACUC information sheet have been developed and will be available to all users. We are also converting the existing Microsoft Word forms to a fillable PDF format.
Are sites required to use the ORPP&E core library?
The ORPP&E core library was designed to provide a standard set of forms and letters for all sites. The Project Cover Sheet is required for all submissions. The IRB Information Sheet is required for all submissions to the IRB (internal and external). However, IRBNet supports the ability for you to upload your supplemental forms (such as if a particular form or template is required to support state or local regulations) via the Library Manager.
Will each institution have specific templates for its forms?
ORPP&E, along with a field-based workgroup, has developed a core library of form and letter templates for each of the committee and subcommittee workspaces in IRBNet. However, IRBNet supports the ability for you to upload your supplemental forms (such as a particular form or template required to support state or local regulations) via the Library Manager. If a change to an existing form is needed, fill out a change request form located on the VAIRRS SharePoint (VA network access only). For modified forms and newly created forms, the draft forms will be posted to the VAIRRS SharePoint portal in the ORPP&E Standard Form Library (VA network access only) for a review period. Notification for all new forms, wizards and letter templates will be included in the monthly VAIRRS newsletter.
Does ORPP&E have guidance on how each smart form/wizard question should be answered?
Researchers must answer simple “Yes” or “No” questions. ORPP&E reviews all feedback on the smart forms from the research community. If a question seems to be producing inconsistent answers or is causing confusion among your researchers, please email the project team at VAIRRS@VA.gov. Users can reference the VAIRRS IRBNet Wizards Navigational Guide (VA network access only) for high-level instructions on how to navigate the online wizards in IRBNet.
Should the OGE Form 450 Alternative-VA be submitted through IRBNet?
No, the OGE Form 450 Alternative-VA should not be uploaded into IRBNet. ORPP&E has requested that the researcher submit the OGE Form 450 Alternative-VA outside of IRBNet. The researcher may attach the findings to their package if the COI review is complete. The new financial COI IRBNet module was deployed in Summer 2022.
Where do investigators, administrators and committee members obtain documents/templates in the ORPP&E Library outside of IRBNet?
Investigators, administrators and committee members should refer to the ORPP&E Standard Form Library (VA network access only) on the VAIRRS SharePoint (VA network access only). The library is broken into three categories: Researcher/Administrator, Committee Members/Reviewers and Summary of CCB Changes. Once a category is selected, documents are further broken down to three folders: Forms & Templates, Letters, and Additional Documents. By selecting the correct library, users assure they are using the most recent template and alleviate the need to maintain a copy of the standard ORPP&E documents.
Where do investigators obtain documents/templates in the ORPP&E Library?
Investigators may access the ORPP&E library using the two methods described below. The research program does not need to maintain the standard ORPP&E documents in the local library.
Method #1
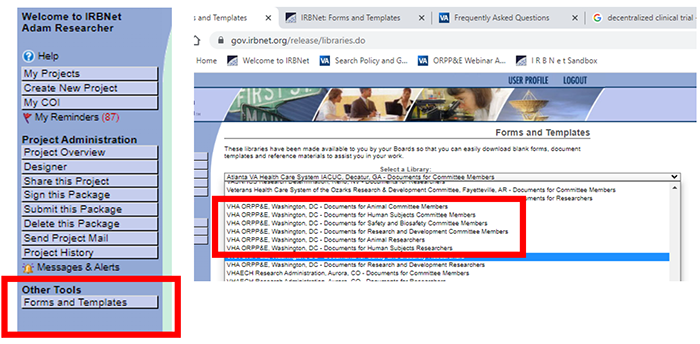
Select “Forms and Templates” from the Other Tools menu.
Select the appropriate VHA ORPP&E library.
Method #2
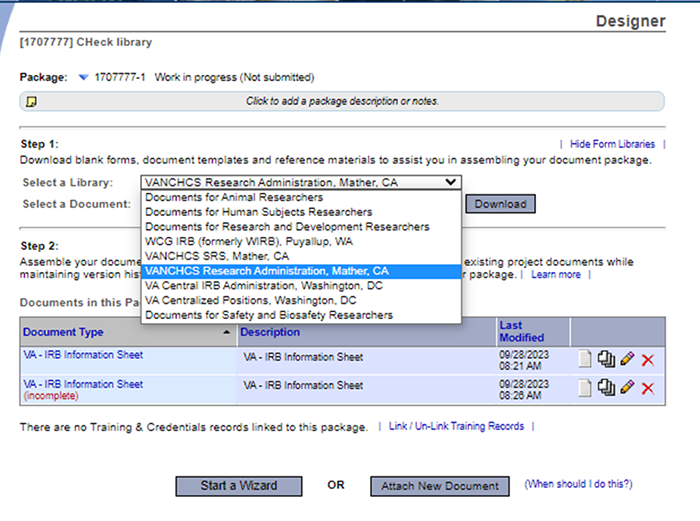
On the Designer page, select the appropriate library in the “Select a Library” drop-down.
Are local sites using IRBNet required to maintain hard copies of research records? For FDA-regulated studies?
No, VAIRRS, VA’s enterprise instance of IRBNet, has an Authority to Operate (ATO) to store VA records and is fully compliant with the FDA requirements at 21 CFR Part 11. The research administration office does not need to download the research records created and stored in IRBNet (VAIRRS) into another electronic storage location or hard copy subject to local and Sponsor policy.
Are documents in IRBNet considered an official “record”?
Yes. While IRBNet is a platform, it has an Authority to Operate (ATO) to store official VA records. There is no need for the research administration office to download records created and stored in IRBNet (VAIRRS) into another electronic storage location subject to local and Sponsor policy.
Is there an instruction manual that can be distributed for researchers and/or administrators?
IRBNet is an intuitive, web-based tool that has instructions and help text available throughout the application. For additional assistance, users can reference various support resources within the VAIRRS University training library (VA network access only). The library is organized to assist with content navigation to help users access resources specific to their individual needs.
Where can newly transitioned sites go for more help once training is complete?
Many resources are available for newly transitioned sites. VAIRRS University (VA network access only) is an excellent resource that allows VA research employees to easily locate and access VAIRRS-specific training materials. You may also request a one-on-one mentor by emailing the project team at VAIRRS@VA.gov. Please make sure all committee administrators are included on the VAIRRS Administrators listserv to ensure receipt of monthly training announcements.
Is the IRB Chair required to sign approval letters at initial or continuing review?
ORD policy does not require that research approval letters be signed. However, there should be some means of ensuring the legitimacy of the letter and the approval date. In cases where an IRB or Privacy Board has approved a waiver of HIPAA authorization, the form must be signed by the IRB or Privacy Board Chair or a designated member of the IRB or Privacy Board.
Is a wet signature required on board approval letters?
No, there is no ORD policy requirement for a wet signature on any document. VHA policy allows for an electronic or digital signature wherever a signature is required. Local policies may restrict you to wet signatures where wet signatures are described in your local policy. Changes to local policies are recommended to accommodate electronic signatures.
Can the certification statements within the Animal Component of Research Protocol (ACORP) be acknowledged with an electronic signature?
Yes, the certification statements in the ACORP may be signed digitally (defined by VA Handbook 6510 as "a specific electronic signature technology that allows the recipient to prove the origin of the document and to protect against forgery") or with a physical ("wet ink") signature - whichever is preferred. What is important to ORD is that the signature is personally applied by the signatory, unique to the signatory, dated and unambiguously associated with the certification statement on the final approved version of the ACORP. A signature on the IRBNet package does not apply to any particular certification statement, and signatures provided only at the time of initial submission do not document acknowledgment of the contents of the final approved version if the IACUC required any changes before approval. To meet VA requirements, the investigator may digitally sign the ACORP using Adobe Reader and upload the signed ACORP to the package in IRBNet for review. If the IACUC requires modifications before approval, the investigator must also sign the revised version. After the ACORP has been approved with no further modifications, the certification statements on the final approved ACORP (already signed by the investigator) must be signed by the IACUC chair, attending veterinarian and any others whose signatures are required for applicable appendices. The IACUC administrator should publish the approved, signed ACORP as a board document.
Please note that VA now requires the signatures in the ACORP for every protocol documented on the ACORP form (see Guidance Document AR2020-001: https://www.research.va.gov/programs/animal_research/guidance.cfm).
Are coordinators required to sign every package they submit?
Yes, every package must be signed in IRBNet using the “Sign this Package” feature by the principal investigator (PI) or by the submitter on behalf of the PI.
Our site has installed the “Skype for Business” add-on for Internet Explorer. Can we use this add-on with IRBNet?
There is a known issue with the “Skype for Business” add-on for Internet Explorer that affects users who create rich-text content that includes phone numbers. In IRBNet, this primarily affects administrative users who draft decision letters in the rich-text editor. Microsoft has not yet released an update to address this issue. If possible, we recommend that staff who edit letters disable the add-on or use an alternate browser.
Is there a flow chart of the VAIRRS submission process that can be shared with the IRB, RDC, Safety, IBC, IACUC committee members and researchers?
Yes, a high-level flowchart of the intended submission process is available for download within the VAIRRS University training library (VA network access only).
Are wizard forms applicable to animal studies?
The Project Cover Sheet wizard is required at initial submission for all studies (animal studies included). The IRB Information Sheet wizard is not required for animal studies.
Does the IRB Information Sheet need to be filled out for animal research?
No, the IRB Information Sheet wizard is only required for studies submitted to the IRB.
How should VA IACUCs record their facility number and address into the IRBNet Minutes Builder to meet the 1200.07 directive requirements?
VA IACUCs should enter their facility number and address into the "Agenda Location" field in IRBNet. ORD and ORO have determined that if the facility number and address are entered into IRBNet in this manner that will satisfy the requirement from the 1200.07 directive
If a package has been unlocked, how does a committee maintain the integrity of a package?
If a package is incomplete and requires additional documents to be ready for review, it may be unlocked and returned to the study team prior to the committee's review. Once the package has been shared with a reviewer, it should not be unlocked. If the committee determines that modifications are required to secure approval, then the modifications or response materials should be submitted in a new package to preserve the original documents.
Do older studies need to complete the Project Cover Sheet wizard?
Older active studies uploaded when the site enrolled in IRBNet should complete the Project Cover Sheet wizard as soon as possible. The IRB Information Sheet wizards should be completed at the next continuing review (CR).
When completing a wizard, what should be done if the package has already been sent along for PO, ISSO and committee review?
Previously reviewed projects should have a completed Project Cover Sheet or IRB Information Sheet. However, when submitting wizards - specifically in response to the ORD Data Call - approved studies may disregard the guidance text published at the end of the wizard.
Is there a process for handling duplicate projects for studies overseen by the CIRB? Can duplicate projects be merged or deleted?
Projects with a CIRB shell and a duplicate local project may be reconciled if there has been no submission using the CIRB shell. Please get in touch with VAIRRS@va.gov for further instructions.
Does the VAIRRS system allow investigators to submit a package directly to a committee?
Currently, the system does not allow a package to be submitted directly to a committee. An administrator should review a package for completion before sending it to a committee.
Can a package be unlocked for a single committee, but locked for other committees? Do packages operate independently?
No, if a committee unlocks a package, it is unlocked for all committees.
Once a package has been submitted, is it possible to update package forms and documents?
Packages are automatically locked upon submission. The researcher would only be able to modify documents in a package after submission if a committee unlocks the package. They can, however, always submit revised or updated documents in a new package.
How does a user update a study in IRBNet if staff leave the project?
For studies under the oversight of the Central IRB, users can list staff that have left the study in 115 section II. Section II has various areas in the 115a PISC Continuing Review Application and the 115b Local Site Investigator (LSI) Continuing Review Application to list staff that have left the study. For non-Central IRB studies, please follow local guidance for required form updates. All studies must update the personnel section of the Project Cover Sheet wizard to remain consistent with current staffing.
How can I update a principal investigator (PI) for a project in IRBNet?
The PI or a study team member with full access can change the PI in the next package by selecting the “Edit” button on the Project Overview page. The PI’s name cannot be changed on previously submitted packages. Users can reference the PI Change Instructions User Guide for further guidance (VA network access only).
In IRBNet, when a study is renewed, should the initial approval date be changed to the most recent renewal date?
The initial approval date represents the date the project received its first approval from the oversight board. The initial approval date should not be changed or updated when recording renewals.
Do committees have the same set of keys as IRB administrators?
The committee and administrator tools may differ depending on the user's role and level of access.
What is VAEDA (VA Electronic Determination Aid)?
VAEDA is a new, innovative system, available to all VAMCs, created by the ORD to standardize and streamline processes within VA research. VAEDA is a decision support tool created to reduce variability in regulatory determinations for proposed research, quality improvement, program evaluations and innovation projects. To learn more about VAEDA, click here.
Is VAEDA an IRBNet update?
No, VAEDA is independent of IRBNet.
Am I allowed to use a non-VA email address as my recovery email in IRBNet?
Yes, ORD recommends that all IRBNet users utilize a non-VA email address as their recovery email to help ensure continued seamless access to IRBNet when not logged in to the VA network. Your recovery email is used to receive the multi-factor authentication (MFA) code sent to you when you access IRBNet for the first time from a new device. Since IRBNet generates this code, this code is not VA data, and receiving this code to your non-VA email address does not violate VA policy.
How can I view a researcher's current training?
There are several ways to get this information. The first option is to use the Track Training tool. When searching for a user in the Track Training tool, select "Set Dates as of Today." This will pre-populate your search criteria so that it only looks for current trainings. The second option is to use the "Project Team Tracking" section of the Submission Detail page. This section lists all of the researchers on the project and any accepted training records for types that you have flagged. For example, if a researcher is required to take a given CITI course, then you can flag that course on the Manage Types and Flags page, and any time you open the Project Team Tracking section, any researchers that have current training for that course will have a colored flag next to their name. The third option is to use a Training Insight Report. This will give you all of the training records accepted by your research program, which you can then filter for current training. This last option is primarily helpful for reporting across the entire program, instead of an individual project (such as, if you want to know how many people have taken a given course).
Where do we get the VA Central IRB study number (the 4-digit number)?
The number (two-digit year-sequency number) is assigned by the VA CIRB office and is added to IRBNet once it is assigned. The number is inserted as the "Ref #" next to the submission date on your Submission Manager page.
How does a new staff member gain access to IRBNet?
New staff members must register through lRBNet. The local IRBNet administrator must send an email to IRBNet Support at govsupport@irbnet.org for new staff members to request administrator access to the appropriate workspace(s). For user instructions on how to register, users can reference various support resources within the VAIRRS University training library (VA network access only).
Are research coordinators able to see documents associated with a project?
If a project is shared with a user, the user should have "read access" to all documents associated with that project. Read access allows the user to view the documents associated with a project. However, the user will not be able to modify documents or submit a package.
Is the Submission Manager only available to IRB administrators?
The Submission Manager is available to administrators and reviewers. It is not available to study coordinators or study PIs. If a facility does not have a Submission Manager and thinks they should, please reach out to the IRBNet Support contact at your facility or email govsupport@irbnet.org.
For a Central IRB study, how does a PI submit a package or project only for local review (not for the lead site or other sites to review)?
A PI or study team member should create a new package within the project and submit directly to the research committee at their particular site, instead of to VA Central IRB administration.
When setting up studies in IRBNet, should all study team members be listed?
Yes, all study team members should be shared on the project to ensure their training records are linked.
How can a user request IRB reports to be generated and delivered monthly?
Administrators can email govsupport@irbnet.org to request IRBNet insight reports.
How does one access the Field Staff Dashboard?
VAIRRS has published Power BI Dashboards for every medical center in the VA system. To request access to the Field Staff Dashboard, fill out the request form (VA network access only) located on the VAIRRS SharePoint site.
How are new user requests processed through IRBNet?
If the individual is a new user, they can go to VA’s instance of IRBNet, VAIRRS, and create an IRBNet account. If the individual is working to gain access for a research office staff member at their site, then the local IRBNet administrator would email IRBNet Support (govsupport@irbnet.org) to request the individual be granted access to the relevant workspace(s).
For multi-site studies, to which site should the Project Cover Sheet be submitted?
For studies under the oversight of the VA Central IRB, the Project Cover Sheet should be submitted to the VA Central IRB for the lead site and each local site.
For multi-site studies with a Central IRB shell and a duplicate local project, is the Project Cover Sheet required for both the Central IRB and local versions?
No, we are reconciling the list of active projects to account for studies with two versions in IRBNet. The Project Cover Sheet should be submitted to the Central IRB using the Central IRB shell.
How do I close a project in IRBNet?
What is the purpose of the Project Cover Sheet (PCS) wizard?
The PCS wizard is what drives a majority of the metrics needed for the research enterprise dashboards. The data collected in the wizard feeds the local and ORD dashboards. The Chief Research and Development Officer (CRADO) currently utilizes the ORD-level dashboard to view research activity at a national level. The facilities participating in the Dashboard Focus Group may access their dashboard to view research activity at their facility. Dashboards for all medical centers were deployed in May 2022. The ORD dashboard is a decision-making tool that allows ORD to identify gaps in the national research program. Prior to VAIRRS, ORD did not have a central data collection tool that included data on ORD-sponsored, externally funded and locally funded projects. VAIRRS now provides a complete picture of research activity across the VHA research enterprise. Numerous groups within the Central Office are partnering with ORD to use the data provided by VAIRRS to decrease the burden on research offices as it relates to reporting. The end goal is to no longer need to conduct data calls out to the field.
If a researcher submits a Project Cover Sheet wizard and information is found to be incorrect, can the research team go back to the wizard and correct the information? How can this be accomplished without having to generate a new wizard?
The researcher has two options. First, if the Project Cover Sheet wizard is already submitted and the package is locked, the researcher can clone a previously completed wizard and jump to the section that needs to be revised. The second option is if the package is unlocked, and the researcher was instructed to revise it. There is a pencil icon next to the wizard on the “Designer” page for the package to allow them to edit the wizard for resubmission.
Which study team personnel should be included in the Additional Personnel section of the Project Cover Sheet wizard?
At a minimum, the principal investigator, study coordinator, and study team members for whom training is tracked in IRBNet or require access to the electronic health record (EHR) should be included in the study team section of the Project Cover Sheet. The personnel information provided in the Project Cover Sheet wizard should be consistent with any local personnel form that is required for the study action.
On the “Project Personnel” page within the Project Cover Sheet (PCS) wizard, how do users utilize the “Adding, Removing, Editing or No Change” functionalities?
The "Adding, Removing or Editing" question in the PCS enables users to specify if new personnel have been added to a study, if personnel have been removed from a study and/or if other personnel information needs to be updated. If none of these options are applicable (i.e., no changes are needed in this iteration of the form), users may select "No Change." Users completing the PCS wizard for the first time should select the “Adding” option to list personnel.
The data collected in this section will also be used by the ORD Strategic Initiative for Research & EHR Synergy (OSIRES) team to provision access at the time a user’s VA medical center (VAMC) transitions to the Cerner Electronic Health Record.
Please note that so long as an individual is listed on the PCS as “additional personnel,” their name and information will appear on the PDF, regardless of the option selected for the "Adding, Removing or Editing" question. An individual will only disappear from the PDF once they have been entirely removed from the form via the red "X" icon located at the top left of their name on the Additional Project Personnel page.
Alternatively, while editing the PCS, a user may indicate to the board that they are adding a new individual to the study by selecting the "Adding" option. The new individual and their information will appear on the PDF. If a user has previously entered user information (i.e., a new project role) that needs to be updated on the PCS, the user may select the "Editing" option to update that individual's information.
When should the Project Cover Sheet – Additional Project Personnel section be updated if the change does not require an amendment?
The Project Cover Sheet (PCS) Additional Project Personnel section must be consistent with local requirements and must be kept up to date with project amendments. As electronic health record (EHR) access is required to be changed, the PCS Additional Project Personnel section must be updated to ensure continuation of appropriate EHR access. If a study team member change does not require an amendment, the Additional Project Personnel section should still be updated to reflect the study team member change and submitted to their local research administration in IRBNet. PCS updates for study team member changes that do not require an amendment must not include changes to study details (i.e., study characteristics), as additional changes may require an amendment and oversight committee/subcommittee review. A user must follow the guidance of the oversight committee and/or subcommittee(s) for reporting changes in personnel that do not require an amendment.
Please note, all study team members should be included in the “PCS Additional Project Personnel” section and shared on the IRBNet project to ensure training can be tracked in IRBNet.
Within IRBNet, some CIRB studies have an expiration date, but no report due date; some have a report date, but no expiration date; and some have neither of those dates. What does this mean for each project?
An expiration date indicates that continuing review is required. Reminder notices will go out at 90, 60 and 30 days prior to the expiration date. The continuing review is due into IRBNet 60 days before the expiration date. “Report due date” only means that an Annual Status Report is due from the PISC Study Team. The Annual Status Report is due 30 days before the due date. No dates would indicate that a local site does not require continuing review.
How do we know which studies will require continuing review versus just the annual report?
The initial approval letter will state whether or not continuing review is required. Your initial approval letter will note that continuing review is unnecessary if the study is minimal risk, adheres to the 2018 Common Rule and meets all other criteria. For studies under the oversight of the Central IRB, the approval letter will be specific and provide the individual with the form number: Form 130 Annual Status Report. Some studies are minimal risk and typically do not require continuing review; however, should the Central IRB reviewer or the Central IRB want to see that study, it will be stated in the initial approval letter and continuing review approval letter if an additional continuing review is needed. This is true even if only for one cycle of continuing review.
Why did ORO decide to suddenly develop the VAIRRS/IRBNet RCO Workspace?
ORD and ORO started discussions in July 2021. The VAIRRS contract modification was awarded in April 2022 and formally announced shortly thereafter. The VA contracted for the RCO module in response to feedback from RCOs that publishing audit results, notifying committees and researchers of audit results, and aggregating the data for the Facility Director Certification report was a burden on RCOs.
Is the use of the VAIRRS/IRBNet RCO Workspace required?
For IRBNet to provide an RCO with the aggregate data to support the FDC Part B, the RCO must record all of their audits for the reporting cycle within IRBNet using the electronic tools. Any mandate requiring the use of IRBNet would come from ORO.
Will the new module create more work for the RCOs?
No, the RCO module is expected to reduce the amount of work required by RCOs to record and share audit results in IRBNet.
What are the benefits of the RCO module?
RCOs will be able to easily and automatically access the electronic records they need, record audit results using the electronic audit tools, and automatically have VA committees and researchers properly notified and provided with access to the audit results. At the end of the 2022-23 reporting cycle, IRBNet will provide RCOs with aggregate data supporting that cycle's Facility Director Certification (Part B) report.
Will RCOs be required to use the new module?
For IRBNet to provide an RCO with the aggregate data, and for the data to be accurate, the RCO must record all of their audits for the reporting cycle within IRBNet using the electronic tools. The oversight committee may request RCO audits be submitted using RCO module. Use of the new module is strongly encouraged by ORO.
Are projects required to be shared with all project team members?
As part of each review process, the subcommittee must ensure that the project has been shared with all project team members. This is important because it affects an RCO's ability to access the complete project record and training information when performing an audit.
Who has access to the RCO audits within VAIRRS?
RCOs have the capability to publish an audit report internally or externally. When published internally, the audit report is only available to the individual that published it and other auditors from that site. When published externally, the audit report is available to the study teams and review committees. Publishing externally triggers an email notification to the study team and review committee. Sites can reference the IRBNet Notes – Enterprise Audit Tools (VA network access only) to address any other questions surrounding publishing.
What is VAIRRS University?
VAIRRS University is a revised training library designed to promote self-education and independent learning. This new platform allows VA research employees to easily locate and access VAIRRS-specific training resources. The library contains a variety of materials, best practices and tutorials developed by the VAIRRS Support Team and other VA medical centers (VAMC) that work within VAIRRS, as well as content generated by IRBNet and courses from VA’s Talent Management System (TMS).
How can VA research employees access VAIRRS University?
VA research employees can access the VAIRRS University training library on the VAIRRS SharePoint portal by selecting VAIRRS University from the menu on the left-hand side of the page.
How can users access training resources relevant to their role within VAIRRS?
VAIRRS University is organized to assist with content navigation and to help users access resources specific to their individual needs. Users can select from seven user role pathways and five training material categories to view curated selections of resources available to them.
|
User Role Pathways
|
Training Material Categories
|
Who manages VAIRRS University?
The VAIRRS Support Team manages VAIRRS University, updating existing content and adding new content, as needed, to enhance and improve the learning experience for VA research employees.
Who can answer questions about VAIRRS University?
Please contact the VAIRRS Support Team at VAIRRS@va.gov with any questions about VAIRRS University.