IRB Relationships in the VA: Single IRB Exceptions, Independent (Commercial) IRBs, and changing IRB reliance by the VA Facility
Since January 20, 2020, the VA has required that non-exempt human subjects research be approved or transitioned to follow the requirements in the 2018 Common Rule. This includes a requirement that muti-site research be approved by a single IRB if:
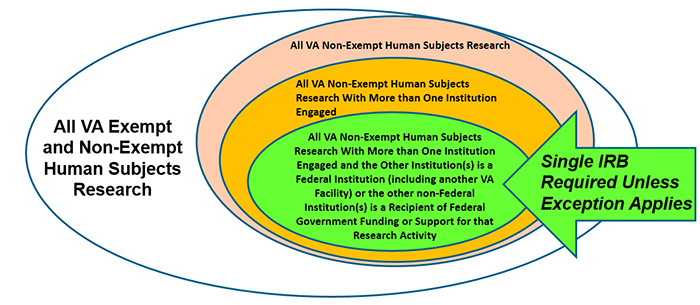
Please note: The cooperative research provisions (also commonly referred to as the “single IRB” requirement) only applies to those institutions that follow the 2018 Requirement. For example, if VA was conducting an industry-funded clinical trial with 14 universities, 3 VA Facilities, and 2 Department of Defense (DoD) Facilities with no other federal support or funding involved, the VA and DoD Facilities are required to use a single IRB unless an exception applies.
Implementation of the Single IRB Requirement in the VA
- ORD supports use of a single IRB when possible, but ORD also recognizes that mandating use of a single IRB in all cases is not logical or feasible. The §.114 cooperative research provision permits VA to make exceptions for use of a single IRB for VA Facilities when ORD determines and documents that use of a single IRB is not appropriate for the particular study.
- VHA Facilities wishing to request an exception from the single IRB requirement from ORD for an applicable study must go to the following SharePoint page (accessible only within VA network): ORD Single IRB Exception Requests - Home (sharepoint.com)
- This page describes who can make a request, how to make a request, how to edit a submitted request, and when to expect ORD’s determination. If you have reviewed that information and still have a question, please email: IRBRelianceandSIRBExceptions@va.gov.
Requesting an Exception from the Single IRB Requirement for Cooperative Non-Exempt Human Subjects Studies
ORD supports use of a single IRB when possible, but ORD also recognizes that mandating use of a single IRB in all cases is not logical or feasible. The §.114 cooperative research provision permits VA to make exceptions for use of a single IRB for VA Facilities when ORD determines and documents that use of a single IRB is not appropriate for the particular study.
VHA Facilities wishing to request an exception from the single IRB requirement from ORD for an applicable study must go to the following SharePoint page (accessible only within VA network): Single IRB Exception Requests (sharepoint.com) (VA network access only)
This page describes who can make a request, how to make a request, how to edit a submitted request, and when to expect ORD’s determination. If you have reviewed that information and still have a question, please email: IRBRelianceandSIRBExceptions@va.gov.
Requesting Change in IRB Arrangements
- ORD policy requires that the VA Medical Center Director request approval from the Chief Research and Development Officer (CRADO) approval when the VA facility wants to establish a new HRPP, change its IRB(s) of Record, or wants its internal IRB to serve as an IRB of Record for a non-VA entity if allowed by ORD policy.
- VA Facilities wishing to change IRB arrangements for a single or multiple studies must submit the ORD application form: Institutional Review Board (IRB) Reliance Request Form.
- The form must be submitted by the VA Medical Center Director via email to the IRB Reliance and SIRB Exceptions email box at (IRBRelianceandSIRBExceptions@va.gov).
- Any additional individuals the form must be submitted to are included in the directions on the application.
Please note The Institutional Review Board (IRB) Reliance Request Form cannot be used by VA Facilities requesting to start a research program. A dialogue with both ORO and ORD is required before any documentation is sent by a VA Facility wishing to start or re-initiate a research program.
Requesting Change in Institutional Review Board (IRB) Arrangements: Commercial IRB
- Only commercial IRBs vetted and approved by ORD, and with which ORD has executed a Master Services Agreement may be used by VA Facilities.
- ORD policy requires that the VA Medical Center Director request approval from the Chief Research and Development Officer (CRADO) when the VA facility wants to establish a new Human Research Protection Program (HRPP), change its IRB(s) of Record, or wants its internal IRB to serve as an IRB of Record for a non-VA entity if allowed by ORD policy. This applies to any change or addition in a VA Facility’s HRPP, including use of commercial IRBs.
- VA FACILITIES MUST NOT CONTACT ANY COMMERICIAL IRBs DIRECTLY TO ENTER INTO THEIR OWN SEPARATE CONTRACTS. THIS IS PROHIBITED BY ORD.
- You will be asked to identify a VA Facility Liaison to be a contact between the commercial IRB and your VA Facility. The role is like the VA Site Liaison for the VA Central IRB.
Application Process for Use of a Commercial IRB Approved by ORD:
- The VA Research Office (Not the Investigator) must send an email that includes (as cc) the Medical Center Director to: IRBRelianceandSIRBExceptions@va.gov.The email must include the following information:
- Name of the Commercial IRB
- Name of the VA Nonprofit Corporation (NPC)
- Statement indicating whether this is a time sensitive request (e.g., your VA Facility has already been selected by the sponsor for a study that is using the commercial IRB)
*If the request for reliance on a commercial IRB is time-sensitive (e.g. the sponsor requires the VA Facility to enroll the first participant within one week of site selection), email and/or call:
Note: Do not submit an Institutional Review Board (IRB) Reliance Request Form to ORD for a commercial IRB reliance request.
- When the commercial IRB reliance request is received, ORD and ORO will send your VA Facility instructions to complete the following templates:
a. Commercial IRB Authorization Agreement (only fields to complete are the name of the VA facility, Federalwide Assurance (FWA) number, and the electronic signatures of the Facility and NPC Institutional Officials); and
b. Local template for standard operating procedures (SOPs) to modify for use of the commercial IRB
Note: Submit both documents together when you have completed them to IRBRelianceandSIRBExceptions@va.gov.
- ORD will send you a letter approving or not approving the use of the commercial IRB. If approved, ORO will inform your VA Facility when the SOP is complete.
VA Facility Commercial IRB Endorsement Letter Requirement
To help ensure that the VA Facility’s research office is aware of any VA study submitted to an ORD-approved commercial IRB, an endorsement letter must be signed by the ACOS/R&D, AO/R&D, or VA Facility’s commercial IRB liaison and included with the submission packet for the study. Please go the following links for addition information about this requirement and the endorsement letter template:
Current List of Commercial IRBs Approved by ORD for VA Facilities as of September 25, 2020
- Advarra
To set up an account with Advarra to use CIRBI, please follow the CIRBI Quick Steps instructions. For additional assistance contact the Advarra CIRBI helpdesk between 8am - 8am ET send an email to CIRBI@advarra.com or call 1-866-99CIRBI (1-866-992-4724).
- Sterling IRB
To set up an account with Sterling to use the SilverLink portal, please follow the SilverLink Quick Start Guide. For questions regarding the SilverLink portal, please email Software Support at support@sterlingirb.com. For questions regarding the initial submission process, please contact Debby Hybart (Debby.Hybart@sterling.com) in the Site Support Department. For additional information about Sterling IRB visit https://sterlingirb.com/.
- WIRB-Copernicus Group (WCG)
To set up an account with WCG, please follow the instructions located on the VAIRRS Share Point site (VA network access only). The VA investigator or study team member with full access to the project should be able to "share" the project with the VA Facility Privacy Officer and ISSO. For additional assistance contact WIRB at VAreliance@wirb.com.
VA Specific Requirements for Informed Consent and HIPAA Authorizations for Commercial IRB Submission
Any IRB reviewing VA research must follow VA's requirements. The tables below, with language for VA informed consent and VA HIPAA authorizations, were provided to commercial IRBs for them to review and approve VA research. Before submitting, VA Facilities are required to insert VA informed consent language into the commercial IRB’s approved informed consent document. If the study is industry-funded, please note that specific 2018 Common Rule informed consent requirements must also be included if the model consent forms do not contain them. It is the responsibility of the VA Facilities to include the VA Specific language and 2018 Common Rule language prior to uploading their local VA Facility informed consent document or combined informed consent and HIPAA authorizations as part of the application process. If you are using the standalone HIPAA Authorization Form for Research (VA Form 10-0493), please do NOT submit this form for review to the Commercial IRB as it only needs review and approval by your Privacy Officer. A checklist has also been developed by ORD to assist VA Facilities with preparing the informed consent documents and combined informed consent documents/HIPAA authorizations; please do not include the checklist with the commercial IRB submissions for the study.
VA Specific and Selected 2018 Common Rule Informed Consent Requirements When Using an Independent-Commercial IRB Revised 06/28/2023
VA HIPAA Authorization Requirements When Using an Independent (Commercial) IRB Revised 06/28/2023
Checklist for VA Facilities Using Independent Commercial IRBs-ICDs and Combined ICD-HIPAA Authorizations Revised 06/28/2023


