Office of Research & Development |
 |
PERSIAN GULF
VETERANS COORDINATING BOARD
RESEARCH WORKING GROUP MEMBERS
Department of Defense:
Robert E. Foster, Ph.D.
John Mazzuchi, Ph.D.
Lt. Col. James R. Riddle, USAF, BSC
CAPT Michael E. Kilpatrick, MC, USN
Department of Veterans Affairs:
John R. Feussner, M.D. (Chair)
Timothy Gerrity, Ph.D.
Han Kang, Dr. P.H.
Frances Murphy, M.D., M.P.H.
Department of Health and Human Services:
CDR Drue Barrett, USPHS
CDR Patrick McNeilly, USPHS
M. Moiz Mumtaz, Ph.D.
Sheila Newton, Ph.D.
Environmental Protection Agency:
Robert McGaughy, Ph.D.
TABLE OF CONTENTS
EXECUTIVE SUMMARY
*New Research Results *
Funding of Federal Research *
New Research Projects, Initiatives, and Milestones *
Research Priorities *
I. INTRODUCTION *
II. RESEARCH RESULTS IN 1998 *
New Research Publications *
1. Brain and Nervous System *
2. Pyridostigmine Bromide *
3. Symptoms/General Health *
III. RESEARCH FUNDING TRENDS *
1. Overview *
2. Research Funding *
3. Diversity of Research Approaches *
4. Government and Non-Government Researchers *
IV. NEW RESEARCH PROJECTS, INITIATIVES, AND MILESTONES *
A. New Research Projects *
1. Two New Treatment Protocols *
2. New Research Projects from DoD Broad Agency Announcement *
3. VA/DoD Neurobiology of Stress Initiative *
4. Other New Initiatives and Research Related Initiatives *
B. VA National Survey of Persian Gulf Veterans and their Family Members:
Phase III *
C. New DoD Research Initiative *
D. Strategies for Future Deployment Health *
1. National Science and Technology Council (NSTC) *
2. National Academy of Sciences/Institute of Medicine *
3. Military and Veterans Health Coordinating Board *
E. HHS Report to Congress on Multiple Chemical Exposures and Gulf War
Veterans Illnesses *
F. NAS/IOM Assessment of the Health Effects Associated with Exposures
During the Gulf War *
G. Measuring the Health of Gulf War Veterans. *
H. New DoD Center for Deployment Health *
I. VA Center for War-Related Illnesses *
J. Gulf War Veterans’ Illnesses Research Advisory Committee *
K. 1998 Medical Defense Bioscience Review: Low-Level Chemical Agent
Research *
L. 1998 Conference on Federally Sponsored Gulf War Veterans’ Illnesses
Research *
V. RESEARCH MANAGEMENT *
A. Overview *
B. Oversight of Research *
1. Institute of Medicine/Medical Follow-up Agency (under contract to VA and DoD): Health
Consequences of Persian Gulf Service *
2. Research Advisory Committee on Gulf War Veterans’ Illnesses *
3. Executive Office of the President: Presidential Advisory Committee on Gulf
War Veterans’ Illnesses (PAC) *
4. Other Oversight *
C. Research Coordination *
VI. Research Priorities *
A. Research on Treatments for Gulf War Veterans’ Illnesses *
B. Longitudinal Follow-Up for Gulf War Veterans’ Illnesses *
C. Disease Prevention *
D. Improved Hazard Assessment *
E. Departmental Responses *
VII. REFERENCES *
VIII. BIBLIOGRAPHY *
APPENDICES
A.Federally Funded Research Projects
B. Presidential Review Directive -- 5 Executive Summary
C.1998 DoD Broad Agency Announcement Solicitations for New Research
D. Military and Veterans Health Coordinating Board Charter
E. Plenary Session Summaries from the 1998 Federally Sponsored Gulf War Veterans'
Illnesses Research Conference
F. Program and Registration Information for the 1999 Federally Sponsored Gulf War
Veterans' Illnesses Research Conference
G. Treatment Trials for Gulf War Veterans
Introduction
On August 31, 1993, in response to Section 707 of Public Law 102-585, President William J. Clinton named the Secretary of Veterans Affairs (VA) to coordinate research funded by the Executive Branch of the Federal Government into the health consequences of service in the Gulf War. Section 104 of Public Law 105-368 (1998) expands the responsibilities of the Secretary of the Department of Veterans Affairs. VA carries out its research coordinating role through the auspices of the Research Working Group (RWG) of the Persian Gulf Veterans Coordinating Board (PGVCB). The Secretaries of the Department of Defense (DoD), Health and Human Services (HHS), and VA chair the PGVCB.
As part of its coordination role, VA is required to submit to Senate and House Veterans’ Affairs Committees an annual report on the results, status, and research priorities for the year covered by the report. This document, the 1998 Annual Report to Congress, is the fifth report on research and research activities.
This edition of the Annual Report to Congress reports on research funded by federal and non-federal institutions. All new peer-reviewed reports of high quality research, regardless of funding source, add to and build upon existing knowledge. Unpublished research results may be discussed with the anticipation of publication in the open peer-reviewed literature. However, the use of non-peer-reviewed results is done with caution because it does not have the weight of peer-reviewed research results.
This six-section Annual Report highlights and summarizes research progress since the last Report by analyzing the Federal Government’s portfolio of research on Gulf War veterans’ illnesses, highlighting significant research and research-related events and milestones, discussing the management of federal Gulf War veterans’ illnesses research programs, including research oversight, peer-review and coordination, and articulating priorities for future research. In addition, important and relevant background information not strictly research –related, is provided to help better understand the overall context related to Gulf War veterans’ research issues.
back to top
Since the Annual Report for 1997, the earlier investment in research has seen the maturation of a number of new research studies that will bring insight into the health problems of Gulf War veterans. In addition, preliminary findings reported at scientific meetings have influenced efforts to develop reliable models for understanding the illnesses reported and observed.
It remains important to note that all research studies have strengths and limitations. Among the limitations, epidemiological studies are frequently subject to a variety of biases. In particular, studies that rely on self-reported symptoms and exposures are subject to recall bias, and studies that rely on self-selected cohorts (such as registry participants) are subject to selection bias. Biases can distort the magnitude of differences between cohorts and affect the strength of associations between exposures and outcomes. Other factors potentially affecting epidemiological outcomes include sample size and response rate.
Research using animal models is also subject to limitations in its applicability to a specific situation for humans. Sources of limitations include extrapolation of biological processes from one animal species to another and extrapolation of experimental dosing regimens (route of administration, amount, and duration) in animals to real human exposure situations.
The presence of limitations in a particular study does not necessarily invalidate its findings or conclusions but must be taken into account in evaluating a study’s overall weight and impact. For this reason, the strengths and limitations of each of the following new reports of study findings are cited as a guide for the reader:
Brain and Nervous System
In the present reporting period a paper has appeared evaluating the long-term effects of Operation Desert Storm on the psychological distress of US Army Reserve and National Guard veterans. In a comparison of soldiers who deployed to the Persian Gulf region to those who deployed to Germany and those who did not deploy, investigators found elevated symptom levels among Gulf War veterans which could not be explained by variance attributed to demographics, or current life stress events. The role of petrochemical fires was studied. The authors suggest that further research is needed on soldiers reporting high levels of distress and concern about oil fire exposure.
Pyridostigmine Bromide (PB)
A pharmacodynamic study of PB, the agent used for prophylaxis against a nerve agent which was believed to have been available for employment against soldiers in the Gulf War, provided information on the distribution and metabolism of the agent in the human body. In volunteer subjects results show that both gender and weight influence the action of PB. The effect of the drug correlates closely with its plasma concentration. This study may help in relating dose-response studies performed in animals to humans.
Researchers in France studying guinea pigs, found that heat stress did not alter the ability of Pyridostigmine to penetrate the blood-brain barrier. This is a different finding from previous work in mice suggesting that exercise stress increases the penetrance of Pyridostigmine through the blood-brain barrier. This work is provocative, but also illustrates the problems inherent in working with animal models of human conditions.
Laboratory evidence in rats bred to have reduced levels of the PB scavenger butyrylcholinesterase, suggest that PB exposure could enhance the central nervous system-mediated acoustic startle response in these animals. This suggests that PB may penetrate the blood-brain barrier to elicit CNS effects. After repeated exposure to PB the enhanced startle effect was reduced.
Symptoms/General Health
The effort to organize reported symptoms into a case definition has continued. In a study of Air Force Gulf War veterans a cross-sectional questionnaire was completed by 3,723 currently active duty Air Force service members. In addition, 158 GW veterans were clinically evaluated. From a case definition adopted to facilitate analysis, it appeared that the prevalence of mild-to-moderate and severe cases was 39% and 6% respectively, among Gulf War veterans versus 14% and 0.7% among non-deployed personnel. Among those medically examined, 37% were identified as non-cases, 54% were mild-to-moderate cases, and 8% were severe cases. The investigators identified a chronic multi-system condition that was significantly associated with deployment to the Gulf War but also affected non-deployed personnel.
An epidemiologic study in the United Kingdom is currently in process with an overall design in two stages. Stage 1 is complete. It was a questionnaire survey of United Kingdom Gulf War veterans and a sample of Bosnia veterans with appropriate control groups for each. Stage 2 will cull out individuals who fall above a cut-off defining subject ill health for further study.
The results of Stage 1 show that participants in the Gulf War feel their health to be worse than comparable military personnel not deployed there or who served in Bosnia. The pattern of symptoms appears to be the same in the Gulf, Bosnia, and control groups suggesting that the profile of the illness is not unique to the Persian Gulf. With regard to specific etiologic factors (e.g. petrochemical fumes, vaccinations) both Gulf-deployed and non-deployed showed the same statistical association of a perception of poorer health, but multiple vaccinations, used in Bosnia and the Gulf, were linked to reported health effects in the Gulf group only.
The investigators also conducted factor analysis on the symptom data from their population-based survey of Gulf War veterans, Bosnia conflict veterans, and Gulf War era veterans. Exploratory factor analysis on the Gulf War veterans uncovered three factors that loaded symptoms associated with mood, respiratory system, and peripheral nervous system. Confirmatory factor analysis with the symptom data from the Bosnia conflict and Gulf War era cohorts resulted in a similar factor structure compared with the Gulf War veterans. The researchers cautiously suggest that these results do not support the existence of a unique "Gulf War Syndrome".
Studies of three cohorts of Gulf-era veterans at two VA Medical Centers have been ongoing since service members have returned from the Gulf. Utilizing a health symptom checklist and psychological testing 220 Gulf veterans in one study, 73 Gulf veterans in a separate study, and 50 Germany-deployed subjects have been analyzed. Researchers found that the prevalence of self-reported symptoms was greater in the Persian Gulf-deployed cohorts compared to the Germany cohort. These symptoms included neurological, psychological, pulmonary, and musculoskeletal symptoms. Associations of specific medical symptoms with specific etiologic factors were made.
A study reported in 1996 found no consistent increase in overall risk for hospitalization of deployed Gulf War veterans compared to nondeployed Gulf War era veterans. A question remained as to whether a risk remained undetected due to an inadequacy in the standard classification of diagnoses. A new study was designed to remedy the difficulty. After "recalibrating" the case-finding method, it appeared that active duty Gulf War veterans were not at increased risk for hospitalization for unexplained illness.
A study that focused on the Gulf War Veterans’ Health Registries (maintained by DOD and VA) sought to identify risk factors for Gulf War veterans to seek medical evaluation. Up to February 1997 the study included a combined total of 74,653 registry participants and was compared to data collected from 678,587 Gulf War deployed veterans who had served in all military branches. A number of characteristics were associated with registry participation including but not limited to being older, having a home of record in the southeast U.S., being female, and being hospitalized during the twelve months prior to the deployment period.
A prior epidemiological study had found no consistent increase in overall risk for hospitalization of deployed Gulf War veterans compared to nondeployed era veterans. The study did, however, find an increased risk of hospitalization for testicular cancer among deployed Gulf War veterans during the last 5 months of 1991, just after deployment. The higher risk did not carry over into 1992 or the first nine months of 1993. In a study now completed the hospitalization rates have been tracked to March 1996 to provide information concerning the more long-term risk of testicular cancer in deployed veterans. The new study tracks only admissions to U.S military hospitals. Among other findings the present study confirms that deployed personnel had a greater risk than non-deployed personnel in the early months after deployment. The increased risk persisted, at a constant level, for approximately 3 years. At the conclusion of the follow-up, the cumulative probability of hospitalization was 0.034% for deployed and 0.035% for nondeployed. The data have been modeled in various ways in order to reach certain conclusions. The investigators have observed that the elevated risk of the deployed in the latter part of 1991, which followed the reduced number of cases in 1990 and earlier in 1991, could be ascribed to selection effect and deferred care during deployment.
Another recent study has focused on veterans with fatiguing illnesses drawn from the VA Persian Gulf Registry Program. Seventy-two Registry participants with complaints of fatigue and/or chemical sensitivity who agreed to participate were sent a screening questionnaire to identify subjects who fit a contemporary set of criteria for Chronic Fatigue Syndrome (CFS) and/or Multiple Chemical Sensitivities (MCS). Screening questionnaires yielded provisional diagnoses allocating subjects to one or the other or both disorders. These were followed by careful medical evaluation for the purpose of providing an accurate outcome diagnosis. This yielded a revised allocation of diagnoses in which 15 of the 68 subjects selected for the study were excluded based on psychiatric diagnoses. Eighty one per cent of the remainder retained diagnoses of CFS or MCS or both. Nineteen per cent did not fulfill any case definition. Analysis of the results showed that the screening questionnaire was not predictive of the diagnostic outcome. Veterans were found to be substantially less ill than indicated by the screening questionnaire. In comparison with a nonveteran study population, the same result was found.
The investigators also observed that the rate of MCS in veterans with CFS, compared to the rate of MCS in nonveterans with CFS, was not significantly different in their study. This casts some doubt on the hypothesis of chemical sensitivities arising from exposure to various chemicals during deployment, but one must remember that this was not a population-based study and therefore caution must be taken when interpreting the findings.
The UK Ministry of Defence (MoD) published its findings on a clinical case series of the first 1000 Gulf War veterans who registered in MoD’s Medical Assessment Programme. Using ICD-10 diagnostic coding, investigators were unable to establish a single illness that could account for the variety symptoms reported by Gulf War veterans. Although these observations are useful it must be borne in mind that the subjects were a self-reporting sample of veterans and not necessarily representative of all Gulf War veterans. Thus the data cannot be used to draw any generalized conclusions about Gulf War veterans’ health.
back to top
Appendix A presents the current contents of the Gulf War Veterans’ Research Database that includes 145 projects, and it was last updated during the first quarter of FY’99. Virtually all the current 145 federal research projects directly related to Gulf War veterans’ illnesses started since 1994 are sponsored by VA, DOD, or HHS. The scope of the federal research portfolio is broad, from small pilot studies to large-scale epidemiology studies involving large study populations and major research and academic medical center programs utilizing significant amounts of appropriated research funds.
Currently, the Federal Government is projecting cumulative expenditures of $133.5 million for research from FY’94 through FY’99. Through 1998, 40 projects have been completed, 103 projects are ongoing, and 2 projects have been awarded funds and are pending start-up.
Gulf War veterans’ illnesses research funds finance a broad-based portfolio with respect to research type and research focus. Epidemiology and clinical research each account for approximately one third of the total number of projects, and the remaining third is divided between mechanistic research and development. Since there is a decreasing need for additional studies of symptoms and illnesses, the number of epidemiology projects has become relatively stable and the number of clinical and mechanistic research projects has increased over time.
Thus far, the overall emphasis of research has been greatest in the focus areas of the Brain and Nervous System and in Symptoms and General Health. The third largest investment in research is in the focus area of Diagnosis. These are examined in more detail in the Report.
The number of research projects in the various research focus areas has changed over time since 1994 as a reflection of the evolution of issues centered on Gulf War veterans’ illnesses. Of note is a relatively greater increase over the years of research on chemical interactions, chemical warfare agents and pyridostigmine bromide. These increases are an outgrowth of increased concern over the health risks posed to veterans by exposures to multiple toxic agents at low-levels.
Research sponsored by the Federal Government is conducted at laboratories inside and outside of government. Government researchers conduct less than half of the government-sponsored projects and take advantage of resources unique to the Federal Government and really cannot be duplicated by the non-government sector, e.g. the outstanding in-house military expertise in the area of leishmaniasis research and the large military health and demographic databases. Non-government researchers, primarily university scientists, have been exclusively conducting approximately 30 of the projects. The remaining projects have involved government and non-government consortia of institutions and researchers.
back to top
New Research Projects, Initiatives, and Milestones
Besides new research findings appearing in the scientific literature and at national meetings, there have been several important events since the 1997 Report to Congress and include the awarding of new research projects and the development of new research initiatives including solicitations for new research. Many of the new research projects and initiatives are the direct result of recommendations from a variety of sources including the Presidential Advisory Committee on Gulf War Veterans’ Illnesses (PAC, 1996b), the Institute of Medicine panel on the Health Consequences of Service in the Persian Gulf War (IOM, 1996), and the RWG (PGVCB, 1995, 1996b).
Research Projects:
Initiatives:
IOM:
back to top
Among the many issues that confronted the RWG during 1998, the question of future research priorities was of great importance. Over the course of 1998, the RWG established the following priorities for future research:
back to top
I. INTRODUCTION
On August 31, 1993, in response to section 707 of Public Law 102-585, President William J. Clinton named the Secretary of Veterans Affairs (VA) to coordinate research funded by the Executive Branch of the Federal Government into the health consequences of service in the Gulf War. Section 104 of Public Law 105-368, signed into law in 1998, further clarifies the responsibilities of the Secretary of VA. VA carries out its research coordinating role through the auspices of the Research Working Group (RWG) of the Persian Gulf Veterans Coordinating Board (PGVCB). The Secretaries of the Department of Defense (DoD), Health and Human Services (HHS), and VA chair the PGVCB.
As part of its coordination role, VA is required to submit to Senate and House Veterans’ Affairs Committees an annual report on the results, status, and future directions of research activities. This document, the 1998 Annual Report to Congress, is the fifth report on research and research activities.
This edition of the Annual Report to Congress reports on research funded by federal and non-federal institutions. All new peer-reviewed reports of high quality research, regardless of funding source, add to and build upon existing knowledge. Unpublished research results may be discussed with the anticipation of publication in the open peer-reviewed literature. However, the use of non-peer-reviewed results is done with caution and because it does not have the weight of peer-reviewed research results.
This Annual Report is divided into six sections. Following this section, Section II highlights and summarizes research progress since the last Annual Report. Section III is an analysis of the Federal Government’s portfolio of research on Gulf War veterans’ illnesses. Section IV highlights significant research and research-related events and milestones since the last Annual Report. Section V discusses the management of federal Gulf War veterans’ illnesses research programs, including research oversight, peer-review and coordination. Section VI discusses research priorities established in 1998 for future new research. In addition, preliminary findings reported at scientific meetings have influenced efforts to develop reliable models for understanding the illnesses reported and observed.
back to top
II.
RESEARCH RESULTS IN 1998In the past year there have been several research studies that have yielded results that provide new and expanded information on the health problems of Gulf War veterans. This section provides brief summaries of research projects for which results have become available in the past year. Because all credible scientifically peer reviewed research must be considered in any future science-based assessments of Gulf War veterans’ illnesses, these summaries are inclusive of both federally funded and non-federally funded research. The primary source of information on non-federally funded research is from the peer-reviewed scientific literature. The RWG tracks all federally funded research projects reported to the RWG related to Gulf War veterans’ illnesses. These projects are described in greater detail in Appendix A.
It is important to note that all research studies have strengths and limitations. Among the limitations, epidemiological studies are frequently subject to a variety of biases. For example, studies that rely on self-reported symptoms and exposures are subject to recall bias, and studies that rely on self-selected cohorts (such as registry participants) are subject to selection bias. Biases can distort the magnitude of differences between cohorts and affect the strength of associations between exposures and outcomes. Other factors potentially affecting epidemiological outcomes include sample size and response rate.
Mechanistic Research using animal models is also subject to limitations in its applicability to a specific situation for humans. Sources of limitations include extrapolation of biological processes from one animal species to another and extrapolation of experimental dosing regimens (route of administration, amount, and duration) from animal experiments to real human exposure situations.
The presence of limitations in a particular study does not necessarily invalidate its findings or conclusions but must be taken into account in evaluating a study’s overall weight and impact. For this reason, the strengths and limitations of each of the new reports of study findings are cited as a guide for the reader.
In previous reports to Congress and other publications, research has been categorized according to the particular focus of the research. The research reports summarized below are grouped according to those focus areas, which are defined in Section III-1.
back to top
The Long-Term Effects of Operation Desert Storm on the Psychological Distress of US Army Reserve and National Guard Veterans (DoD Funded)
Stuart and Bliese (1998) report on factors related to the long-term psychological health of a sample of U.S. Army National Guard and Reserve Unit veterans who served during the Gulf War. In 1994 the investigators measured general distress symptoms and correlated and compared them among soldiers who deployed to Germany, the Persian Gulf region, and throughout the United States and with soldiers who did not deploy. Elevated symptom levels found in Gulf War veterans could not be explained by variance attributed to demographics, or current life stress events. Reported exposure and the degree of then current concern due to petrochemical fires in Kuwait were found to be significantly related to elevated symptom measures beyond the effect of combat-zone-related stresses. The authors suggest that a subset of Gulf War reserve veterans continued to have elevated levels of distress related to oil fire exposures approximately two years after the event and indicated that further research is needed on soldiers reporting high levels of distress and concern about oil fire exposure.
back to top
Population Pharmacokinetics and Pharmacodynamics of Pyridostigmine Bromide (PB) for Prophylaxis Against Nerve Agents in Humans (DoD Funded)
This study was conducted to determine the pharmacokinetics and pharmacodynamics of PB given as 30 mg of PB every 8 hours in healthy subjects (Marino, et al, 1998). Plasma PB concentration and red blood cell acetylcholinesterase activity were measured in blood samples collected during a 3-week period. Population analysis was performed using standard pharmacokinetic and pharmacodynamic models. The pharmacokinetic model that best fit the PB plasma levels was a two-compartment open model with first-order absorption, a lag time, and first-order elimination from the central compartment. The pharmacodynamic model that best fit red blood cell acetylcholinesterase activity was an inhibitory Emax model with an effect compartment linked to the central compartment. The results showed that the pharmacokinetics of PB bromide is both gender and weight dependent. The drug effect does not lag significantly from the plasma concentration and returns to near normal within 8 hours, with the blood cell acetylcholinesterase inhibition >10% at the time of the trough. This study may help in relating dose-response studies performed in animals to humans.
Heat Stress, Even Extreme, Does Not Induce Penetration of Pyridostigmine into the Brain of Guinea Pigs (Non-Federally Funded)
To further explore the potential for stressors to alter the blood-brain barrier (BBB) and thus allow Pyridostigmine to pass into the central nervous system, Lallement et al. (1998) studied the effects of heat stress on BBB penetration of Pyridostigmine. A control group of guinea pigs at room temperature (maintaining body core temperature at 39.8 degrees C), and two other groups of animals maintained for two hours each at two different elevated temperatures sufficient to maintain core temperatures of 41.5 and 42.6 degrees C respectively were injected with Pyridostigmine 90 minutes into the exposures. Passage of Pyridostigmine into the brain was assessed by measurements of brain acetylcholinesterase activity and by autoradiography of injected radiolabelled Pyridostimine. Neither assessment method was able to detect a temperature effect on the blood brain barrier with respect to penetration of Pyridostigmine into the brain. Other research on BBB penetration of Pyridostigmine suggested that exercise stress altered the BBB of mice allowing penetration of Pyridostigmine into brain (Friedman, et al., 1996). This research points out the effect varying experimental conditions may have on outcomes, including the use of different animal models. If choice of animal model played a role in the different findings of this study it reinforces the difficulty of extrapolating laboratory results from one animal species to another.
Persistently Exaggerated Startle Responses in Rats Treated with Pyridostigmine Bromide (VA Funded)
Servatius, et al. (1998) were interested in whether low butyrylcholinesterase (BuChe a natural PB scavenger) activity could enhance central nervous system responses to PB by effectively increasing the amount PB available to inhibit acetylcholinesterase (Ache) activity, thereby increasing levels of this neurotransmitter. The researchers compared the startle responses in two strains of laboratory rats – the Wistar-Kyoto (WKY) rat that has low BuChe activity and the Sprague Dawley (SD) rat that has normal BuChe activity. Each set of animals received PB for 7 consecutive days. At 15 days following the initiation of dosing the WKY rats exhibited a PB dose-dependent enhanced startle response, in comparison to the SD rats, from a noise stimulus. The enhanced startle response decreased over a number of days following PB. Acoustic startle data from a second exposure to PB suggest that the effect of PB on startle responses in the WKY rats diminish with repeated exposure. This research shows the potential for CNS effects from PB in persons with reduced BuChe activity. However, it is estimated that a very small fraction (less than 1%) of Gulf War veterans may have had reduced BuChe activity.
back to top
A Chronic Multisystem Illness Affecting Air Force Veterans of the Gulf War (HHS Funded)
Fukuda et al., (1998) attempted to organize symptoms reported by Air Force Gulf War veterans into a case definition, characterize clinical features and evaluate risk factors for symptoms. The investigators utilized a cross-sectional population survey of individual characteristics and symptoms combined with a clinical evaluation. The cross-sectional questionnaire was completed by 3,723 currently active volunteers from four Air Force populations. Additionally, cross-sectional clinical evaluation was performed on 158 Gulf War veterans from one unit, irrespective of health status. A case was defined as having one or more chronic symptoms from at least 2 of 3 categories (fatigue, mood-cognition and musculoskeletal). The prevalence of mild-to-moderate and severe cases was 39% and 6%, respectively, among Gulf War veterans versus 14% and 0.7% among nondeployed. Fifty nine (37%) clinically evaluated Gulf War veterans were noncases, 86 (54%) mild-to-moderate cases and 13 (8%) were severe cases. The investigators identified a chronic multisystem condition that was significantly associated with deployment to the Gulf War but was not associated with specific Gulf War exposures and also affected nondeployed personnel.
A Controlled Epidemiological and Clinical Study into the Effect of Gulf War Service on Servicemen and Women of the United Kingdom Armed Forces (DoD Funded)
This research addresses the prevalence of unexplained illnesses, including chronic fatigue-like symptoms, in members of the United Kingdom Armed Forces who were deployed to the Persian Gulf during the Gulf War, and who have served, or are serving in Bosnia (Unwin, et al., 1999). This study is a two-stage cohort study. Stage 1, which is complete, was a questionnaire survey of Gulf War veterans selected at random, and an equivalent sample of Bosnia veterans, with appropriate control groups for each. Stage 2 will involve interview, examination, and testing of all those (approximately 10%) in stage 1 who fall above a cut-off defining subjective ill health. Information gathered at stage 2 will be used to estimate the prevalence of diagnosed and unexplained morbidity, including chronic fatigue symptoms, in UK service personnel, and to calculate whether there is an excess associated with Gulf War and/or Bosnia service. If there is, then the researchers will be able to examine pre-morbid and psychological factors which may be implicated in such an increase, as well as identify avenues for further research. This epidemiological study should ascertain if service in the Gulf War by UK armed forces personnel was associated with an increase in physical and / or psychosocial morbidity, and if so, if there is evidence of an increase in either new or ill-defined conditions such as chronic fatigue syndrome, or an illness peculiar to Gulf War service.
Stage 1 results show that British service members who served in the 1991 Gulf War and who completed the detailed health questionnaire, feel their health to be significantly worse than comparable military personnel who were not deployed there or who served in Bosnia. Of the approximately 3000 service members studied, most, but not all, have relatively mild disorders and complaints but all symptoms (e.g., headache, fatigue, chest pain, poor concentration etc) and all conditions (e.g., asthma, arthritis, dermatitis etc) were more common; chronic fatigue and post-traumatic stress are about twice as common. The pattern of symptoms, when analyzed, was the same in the Gulf, Bosnia and control groups suggesting, according to the researchers that there is no specific "Gulf War Syndrome" - although this does not mean that sick veterans do not have a genuine illness.
In addition to these findings, the research found that of 29 potential causes of veteran’s illnesses inquired about, all were statistically associated with poorer perceived health in Gulf veterans. Those who did not serve in the Gulf and were exposed to the same things (e.g. petrochemical fumes, routine vaccinations, dealing with casualties, etc.) showed the same statistical association with poorer health. Potential causes of ill-health confined to the Gulf (e.g. protection against chemical and biological weapons) showed the same kind of association with ill health. Multiple vaccinations (used in Bosnia and the Gulf) were linked to reported health effects in the Gulf group only.
Is There a Gulf War Syndrome? (DoD Funded)
In conjunction with their population-based health survey of UK Gulf War veterans, Bosnia conflict veterans, and non-deployed Gulf War era veterans (Unwin et al., 1999 and above), investigators at the Gulf War Research Unit at Guy’s, King’s, and St. Thomas’s Medical School used the data from the survey to conduct exploratory factor analysis to identify underlying factors and describe the factor structure of the symptoms reported in the Gulf War cohort (Ismail et al., 1999). Confirmatory factor analysis was used to test the fit of this factor structure in the Bosnia and Gulf War era cohorts. The investigators found that three factors in the Gulf War cohort together accounted for about 20% of the common variance. According to the symptoms that loaded on the factors, they labeled the factors mood, respiratory system, and peripheral nervous system. The confirmatory factor analysis on the Bosnia and Gulf War era cohorts revealed a similar structure to that identified in the Gulf War cohort. Recognizing the problems of interpretation of these types of factor analytic models, the investigators cautiously suggest that these findings do not support the existence of a unique "Gulf War Syndrome".
Health Status of Persian Gulf Veterans: Self-Reported Symptoms, Environmental Exposures and the Effect of Stress (VA Funded)
Researchers at the Boston VA Environmental Hazards Research Center and the New Orleans VA Medical Center studied three cohorts of Gulf-era veterans. Two of the cohorts were deployed to the Gulf (one which had been followed by Boston VA Medical Center researchers since their members had returned from the Gulf War in 1991 (Proctor, et al., 1998), and the other which had been followed by New Orleans VA Medical Center researchers since their return). The third cohort was composed of members who were meant to deploy to the Gulf, but only deployed as far as Germany.
The Gulf-deployed veterans that were studied were selected from larger cohorts (Wolfe, et al., 1998) by a stratified, random sampling strategy designed to produce an equal representation of higher and lower symptom reporters and to oversample for women. The symptom reporting stratification used the results from a 20 item health symptom checklist (HSC) in which each subject was asked to report the frequency that he/she experienced symptoms over the past several weeks. Each response was scaled from 0 to 3 (0 = none; 1 = a little; 2 = often; 3 = very often). "High symptom reporting units" were defined as those for which > 50% of the members reported > 5 symptoms. "Low symptom units" were defined as those for which >= 50% of the members reported <= 5 symptoms. Likewise, high and low symptom reporting individuals were identified using the same symptom number cutpoint. The Germany-deployed cohort was represented by a sample of a National Guard unit from Maine.
The complete protocol included medical and occupational history questionnaires; an expanded 52 item HSC; several psychometric measures to assess psychological symptomatology (e.g., the Brief Symptom Interview); an environmental interview; a neuropsychological test battery; and psychological diagnostic interviews (including the Clinical Administered Scale for PTSD (CAPS)). The Boston-studied cohort (also known as the Fort Devens cohort) consisted of n = 220 subjects; the New Orleans cohort, n=73; and the Germany-deployed cohort, n = 50).
The expanded HSC was scaled from 0 to 4 for symptom frequency (0 = no symptoms; 1 = 1-2 times in all; 2 = some, 1-2 times/week; 3 = often, several times/week; 4 = very often, almost every day). Each of the 52 symptoms was assigned by four independent judges to one of nine body systems (cardiac; dermatological; gastrointestinal; genitourinary; musculoskeletal; neurological; neuropsychological; psychological; and pulmonary). A body-system symptom score (BSS) for each subject was created as the sum of the ordinal symptom frequency scale (0-4) for symptoms in each system.
The researchers found that the prevalence of self-reported symptoms from the expanded HSC was greater in both of the Persian Gulf-deployed cohorts compared to the Germany cohort. Analyses of the BSS, weighted to account for sampling design, and adjusted by age, sex, and education, indicated that Gulf-deployed veterans were more likely to report neurological, pulmonary, gastrointestinal, cardiac, dermatological, musculoskeletal, psychological, and neuropsychological system symptoms than Germany-deployed veterans. Using a priori hypotheses about the toxicant effects of exposure to specific toxicants, the relationships between self-reported exposures and body-system groupings were examined through multiple-regression analyses, controlling for war-zone exposure and post-traumatic stress disorder (PTSD). Self-reported exposure to smoke from tent heaters was significantly associated with cardiac pulmonary symptom scores; self-reported exposures to pesticides and CBW agents were significantly associated with musculoskeletal scores; and self-reported exposures to debris from Scud missiles and CBW agents were significantly associated with neurological, neuropsychological, and psychological scores. The most prevalent symptoms that were reported were the same in Gulf-deployed and Germany-deployed groups with the Gulf-deployed veterans reporting higher prevalence rates than the Germany-deployed veterans.
As the researchers caution, the relationships between self-reported symptoms and exposures could be the result of attribution bias where people who feel they are sick are more likely to report environmental exposures. However, it should also be noted that associations predicted by a priori hypotheses about known toxicant effects from self-reported exposures were observed. The unresolved nature of the relationships underscores the need for more objective exposure and health outcomes data to draw definitive conclusions regarding causation.
Hospitalizations for Unexplained Illnesses among U.S. Veterans for the Persian Gulf War (DoD Funded)
In a previous epidemiologic study (Gray, et.al., 1996) found no consistent increase in overall risk for hospitalization of deployed Persian Gulf War veterans compared to nondeployed Gulf War era veterans. However a question remained as to whether a risk remained undetected due to the possible inconsistency in classification (using the International Classification of Diseases, 9th Revision, Clinical Modification system: ICD-9) of a new or a poorly recognized syndrome. The present study (Knoke, et al., 1998) compares the postwar DoD hospitalizations for diagnoses consistent with an unexplained illness of Persian Gulf War veterans with their non-deployed counterparts as a means of determining whether a difference in DoD hospitalization risk had been undetected in the previous study. The study used a collection of 77 diagnoses assembled by the Emerging Infections Program, Centers for Disease Control and Prevention as a determinant of study outcome: i.e. whether a record would or would not be included in the study. The results of the study indicate a slightly higher hospitalization risk for unexplained illness among deployed Gulf War veterans than among those not deployed. However, the excess hospitalizations are attributable to participation by some individuals in a DoD program for clinical evaluation the (The Comprehensive Clinical Evaluation Program (CCEP)) rather than for clinical management. After correction for participation in the clinical program, active duty Gulf War veterans were not at increased risk for hospitalization for unexplained illness.
The study conclusion is that, after exclusion of CCEP hospitalizations, deployed Gulf War veterans were not at greater risk than those not deployed. A slightly lower hospitalization risk for deployed than for nondeployed is ascribed to a "healthy service member effect" where those selected for deployment are, on average, slightly healthier than those not selected. Strengths cited by the authors for the study are: (1) large group sizes; (2) availability of numerous group covariates provide high statistical power for detection of differences in the hospitalization risk between groups; (3) discharge diagnoses are recorded and edited by DoD hospitals; (4) and active duty personnel are said to have few opportunities for hospitalization outside the DoD system so that there are few missing values. Limitations that the authors found to affect their study are: (1) probable miscoding of the deployment status of small number of Gulf War veterans; (2) separation of servicemen during the 4.67 years of the follow-up (59.8% of deployed and 54.3% of nondeployed veterans separated by April 1, 1996 and were therefore ineligible for the study population); (3) the broad categorization of deployment status (deployed for 1 or more days during the August 8, 1990 through July 31,1991) may have masked illness resulting from time or geography specific exposures; (4) illnesses that were considered insufficiently serious for hospitalization were not included in the study; and (5) the collection of diagnoses used as the outcome measure in the study was not designed specifically for the purposes of the study.
Gulf War Veterans’ Health Registries. Who is Most Likely to Seek Evaluations? (DoD Funded)
This historical cohort study (Gray, et al., 1998) sought to characterize risk factors for Gulf War veterans to seek medical evaluation. The study used the combined data collected between 1993 and up to February 28, 1997 from the VA Persian Gulf Veterans' Health Registry (VA registry, 52,313 veterans) and DoD Comprehensive Clinical Evaluation Program (DoD registry, 23,654 veterans). Because the population is self-selected, the symptoms and diagnoses do not reflect those of a population-based sample of the entire deployed population and should be interpreted cautiously. The data of the combined total of 74,653 registry participants (10.7% of Gulf War veterans) was compared to the 97.4% of Gulf War deployed veterans (678,587) for whom demographic data was available and who served in Army, Navy, Marine Corps, Air Force, or Coast Guard for one or more days during from August 8, 1990 till July 31, 1991. Demographic data included sex, date of birth, paygrade, race, ethnicity, home of record, service branch, service type, marital status as of 1991, DoD primary occupational specialty, beginning and ending dates of Gulf War Service, and date of service separation. Of combined registry participants, 30.6% consisted of personnel who sought medical attention, with or without symptoms, for whom no clinical diagnoses were evident. The nineteen most frequent diagnoses were similar to previously reported general and nonspecific symptoms. The highest risk for registry participation was for Army personnel, National Guard, reserve, and active duty personnel who separated after the war as opposed to remaining on active duty after the war. Other characteristics associated with registry participation included: being stationed in the Gulf War theatre during the fighting; being older; being enlisted or a warrant officer; having a home of record in the southeast U.S.; being a supply handler; being female, and being hospitalized during the twelve months prior to the deployment period. In addition to quantifying the independent risk factors of registry participation, the study yields the observations that there is increased risk for registry participation for personnel who were in the Gulf War theater during combat periods, who were construction workers, who separated from active duty, and who were hospitalized during the 12 months prior to the deployment.
Testicular Cancer and Persian Gulf War Service (DoD Funded)
In a previous epidemiological study (Gray, et.al, 1996) found no consistent increase in overall risk for hospitalization of deployed Persian Gulf War veterans compared to nondeployed Gulf War era veterans. The study did, however, find an increased risk of hospitalization for testicular cancer among deployed Gulf War veterans during the last 5 months of 1991 which coincides with the period immediately after deployment. The higher risk did not carry over into 1992 or the first nine months of 1993. The objectives of the present study (Knoke, et al, 1998) were to compare rates of hospitalization for testicular cancer in deployed and nondeployed active-duty Gulf War era veterans from the end of the deployment period to March 13, 1996 and to provide additional information regarding the increased risk of testicular cancer in deployed veterans that was noted in the previous study. The study population included: all members of the study population who were on active duty (either Army, Air Force, Navy, Marine Corps or Coast Guard) when the Gulf War ended July 31, 1991, were either deployed to the Persian Gulf War for 1 or more days during the deployment period (August 8, 1990 through July 31, 1991), or were not deployed but were on active duty for at least part of the Gulf War deployment period. Members of the U.S. Reserve and National Guard forces were not included in the study. Only admissions to U.S. military hospitals reported to the DoD computerized hospitalizations database from July 31, 1991 to March 13, 1996 were included. Outpatient visits (of which there was one noted in hospital records) were not included. No evaluation of hospitalizations prior to the conclusion of the deployment period was included. From a population of 517,223 active-duty male personnel deployed to the Persian Gulf War and 1,291,323 Gulf War era male personnel not deployed, a study population of 505 individuals whose records listed a principal diagnosis of testicular cancer were selected. This included 134 deployed veterans and 371 nondeployed veterans. An analysis using a multivariate model analysis including deployment status and the reduced set of covariates was performed on a population of 1,290,494 nondeployed and 517,050 deployed servicemen a significant risk factor for testicular cancer, including all 505 cases of testicular cancer. The analysis indicated deployment status overall was not important (RR = 1.05; 95% CI = 0.86-1.29). A separate model analysis for deployed and nondeployed groups with computation of probabilities of hospitalization for testicular as a function of follow-up time indicates that deployed personnel had a greater risk than non-deployed personnel in the early months after deployment. The increased risk persisted, at a constant level. without rising, for approximately 3 years. At the conclusion of the follow-up, the cumulative probability of hospitalization was 0.034% for deployed and 0.035% for nondeployed veterans.
The analysis substantiates the findings of the initial study. When the analysis was repeated but using January 1, 1990 as the beginning of the follow up to determine if the increase in cases among deployed veterans was related to transient factors resulting from service in the Gulf War, the model indicated that risk for deployed veterans was not elevated (RR = 0.89%; 95% CI = 0.75-1.06). In the second model analysis, the rate of testicular cancer is lower among the deployed than among the nondeployed during the predeployment and deployment periods and then increased after the deployment ended. By the end of the follow-up period, the cumulative risk among deployed (0.038%%) is lower than among nondeployed (0.046%). The elevated risk of the deployed in the latter part of 1991, which followed the reduced number of cases in 1990 and earlier in 1991, could be ascribed to selection effect and deferred care during deployment. Strengths cited by the authors for the study are: large group sizes; and availability of numerous group covariates providing high statistical power for detection of differences in the hospitalization risk between groups. Discharge diagnoses were recorded and edited by DoD hospitals; the DoD health care system provides a centralized source of cases with equal availability of care for the deployed and nondeployed populations; almost all cases of testicular cancer result in at least one hospitalization ensuring that relevant cases are included in the data set; and, though the follow up period was relatively brief, it included several years of the age range when men are at the greatest risk of developing testicular cancer. Limitations that the authors found to affect their study were: separation of servicemen during the follow-up period (59.9% of deployed and 54.3% of nondeployed veterans separated by 1 April 1996 and were therefore ineligible for the study population). Furthermore, broad categorization of deployment status (deployed for 1 or more days during the August 8, 1990 through July 31, 1991) may have masked illness resulting from time or geography specific exposures. Lastly, the study only addressed risk in the 4.67 year period after the conclusion of deployment.
Medical Evaluation of Persian Gulf Veterans with Fatigue and/or Chemical Sensitivity (VA Funded)
Pollet, et al., (1998) reported on a study of veterans with fatiguing illnesses drawn from the VA Persian Gulf Registry Program. Seventy-two Registry participants with complaints of fatigue and/or chemical sensitivity, and who agreed to participate in this study, were sent a screening questionnaire to identify potential study subjects with Chronic Fatigue Syndrome (CFS) and/or multiple chemical sensitivities (MCS). Based on the screening questionnaire study subjects were excluded using criteria from the 1994 CFS case definition. Inclusion criteria for the category of CFS were based on the more stringent 1988 case definition that was modified to assess self-reported symptom severity. Besides the inclusion criteria of the 1988 case definition, CFS subjects also had to endorse the severity of their symptoms at 3 on 0 to 5 Likert scales ranging from none to severe. The latter requirement meant that included CFS subjects had "severe" CFS or CFSsevere. Subjects included on the basis of MCS had to report sensitivity to 5 of 8 listed chemicals, report an unusual sensitivity to everyday chemicals, endorse 2 of 4 possible life style changes due to chemical sensitivity, and not satisfy the criteria for CFSsevere. Subjects who were included under the category of CFSsevere/MCS had to satisfy both sets of criteria. Similar criteria were used to recruit and evaluate 95 nonveterans with CFS for comparison with the veterans with CFS. Four veterans were excluded for medical reasons. Of the remaining 68 veterans, 31 fulfilled screening criteria for CFSsevere, 17 for CFSsevere/MCS, and the remaining 20 for MCS alone. These veterans were extensively evaluated medically for the purpose of providing an accurate outcome diagnosis. Fifteen of the 68 were eventually excluded based on psychiatric diagnoses. Of the remaining 53, 33 received a diagnosis of CFS based either on the relaxed 1994 case criteria (CFS94) or on the criteria for CFSsevere. Fourteen of the 33 with diagnosed CFS also had concurrent MCS using published criteria. Four veterans had "idiopathic chronic fatigue", or CF, which was defined as CFS94 with < 4 symptoms of any intensity. Six veterans satisfied the operational case definition of MCS alone. Two of the latter had concurrent CF and 4 had fatigue that did not produce a substantial decrease in activity. The remaining 10 veterans (19% of the 53 evaluated) did not fulfill any case definition. Of the 53 patients, two received a concurrent diagnosis of fibromyalgia. When clinical diagnoses were compared with screening questionnaire results, it was found that the screening questionnaire was not predictive of the diagnostic outcome. Veterans were found to be substantially less ill than indicated by the screening questionnaire. When the same extensive evaluation was applied to the nonveteran study population, the same result was found. This indicates that self-reporting of symptoms is not stable over time. The reason for this is not known but could be due to self-report symptom amplification, test-retest unreliability, or the natural history of CFS.
The investigators note that the rate of MCS in veterans with CFS compared to the rate in nonveterans with CFS was not significantly different in their study. This casts some doubt on the hypothesis that chemical sensitivities developed as a result of exposures to various chemicals during deployment. It must be stressed, however, that this was not a population-based study of fatigue and chemical sensitivities in Gulf War veterans, and therefore caution must be taken when interpreting the findings. Furthermore, the study sample size was small.
Clinical Findings for the First 1000 Gulf War Veterans in the Ministry of Defence’s Medical Assessment Programe (Non-Federally Funded)
The UK Ministry of Defence (MoD) has been conducting a medical assessment program similar to VA’s Gulf War Registry Program and DoD’s Comprehensive Clinical Evaluation Program. The MoD reported on the results of a case series of 1000 UK veterans who presented to the program between October 11, 1993 and February 24, 1997 (Coker et al., 1999). Outcome measures were diagnoses of veteran’s conditions according to the International Classification of Diseases, 10th revision (ICD-10). This clinical case series found that many Gulf War veterans had a wide variety of symptoms, however there was no evidence of a single illness, psychological or physical, to explain the pattern of symptoms seen in veterans in the assessment program. It should be emphasized that this program and the report cited here does not represent a population-based research effort and results should be approached with caution. The veterans in the Assessment Programme were all self-selected, and it is not known whether the veterans in this study were representative of sick veterans as a group.
back to top
III. RESEARCH FUNDING TRENDSAppendix A comprises the current contents of the Gulf War Veterans’ Research Database. Research projects are grouped according to the Department that is responsible for the conduct or sponsorship of the research.
Each entry in the database includes:
Two descriptors can approximately categorize each research project. The first descriptor is a series of research focus areas. These are research areas that have been defined as being particularly important. The current areas of research focus are categorized as follows:
The number of focus areas has expanded since last year by the addition of four new focus areas: Interactions, Prevention, Treatment, and Diagnosis. Each project is assigned up to three focus areas as categorical descriptors. This allows accounting for projects that cover multiple focus areas. For example, a project on the neurophysiological effects of exposure to sarin in animals would have a focus on the brain and nervous system, and a focus on chemical weapons. The number of focus areas (between one and three) assigned to a project depends on the project itself.
The other descriptor for each project is Research and Development Type. The Federal Government defines Research as systematic study directed toward more complete scientific knowledge or understanding of the subject studied. Within this definition there are two broad categories of research: Basic Research and Applied Research. In Basic Research the objective is to gain knowledge or understanding of phenomena without specific application in mind. In Applied Research the objective is to gain knowledge or understanding necessary for meeting a specific need. All research on Gulf War veterans’ illnesses is virtually, by definition, applied research. Within the category of applied research, each research project on Gulf War veterans’ illnesses uses a method of approach to test a specific research hypothesis. Approaches range in type from mechanistic research, addressing potential biological mechanisms of causation, to clinical and epidemiological research that attempts to ascertain illness prevalence and risk factors. Although precise categorization of research types can be difficult because of overlapping methodologies, Gulf War veterans’ illnesses research projects can be divided into the following general types:
MECHANISTIC RESEARCH: research into underlying mechanisms of diseases and illnesses using in vitro and in vivo models.
CLINICAL RESEARCH: application of an intervention, such as in a controlled drug trial, or use of methodologies such as case-control studies to define disease risk factors.
EPIDEMIOLOGY RESEARCH: includes population-based studies focused on outcomes such as mortality, symptoms, hospitalizations, etc., using devices such as postal surveys, telephone interviews, and records reviews.
In addition to the research on Gulf War veterans’ illnesses, the RWG also tracks development work. In general, development is the systematic use of the knowledge or understanding gained from research directed toward the production of materials; devices; systems; or methods, including design, development, and improvement of prototypes and new processes. It excludes quality control, routine product testing, and production. Within the context of Gulf War veterans’ illnesses, the RWG categorizes activities as development as follows:
DEVELOPMENT: An activity that satisfies the general definition of development described above, and is directed toward new biologically based prevention, intervention, and treatment measures.
The Gulf War Veterans’ Illnesses Research Database catalogs only research and development activities that either directly involves Gulf War veterans, or has been initiated to answer specific questions about risk factors. A case of the latter is a research project using animal models for low-level chemical warfare agent health effects. The database does not account for the vast accumulated knowledge derived from the nation’s investment in biomedical research over the past 40 years.
The Gulf War Veterans’ Research Database only contains research that is federally sponsored. This includes research conducted by federal scientists, as well as that by non-federal scientists supported by federal research funds through grants and contracts. Many early federal research efforts were pilot projects and were not subjected to national, external peer review. These pilot projects were necessary to build toward a more fully developed research program.
Lastly, it is not possible to accurately track research efforts that fall within the private sector or otherwise outside of the purview of the Federal Government. Notwithstanding, the Research Working Group attempts to stay abreast of all research relevant to Gulf War veterans’ illnesses. The Research Working Group accomplishes this by monitoring the peer reviewed published scientific literature, attending scientific meetings, and even using newspaper reports and personal accounts of researchers.
Regardless of the entity that supports particular research projects, the Research Working Group will seek to ensure that all research that has undergone rigorous external peer review and has been published in peer reviewed scientific literature will ultimately be used in formal assessments of the nature and cause(s) of Gulf War veterans’ illnesses.
The following sections provide a quantitative overview of the current research portfolio on Gulf War veterans’ illnesses and the evolution of the portfolio over time since 1994. Topics that are covered include overall research expenditures from 1994-1999 (projected), and the types and areas of research in which the Federal Government has invested.
back to top
Virtually all current federal research projects directly related to Gulf War veterans’ illnesses are sponsored by VA, DOD, or HHS. From 1994 through FY’98, the Departments have sponsored 145 distinct research projects on Gulf War veterans’ illnesses. This does not include research projects that recently have been selected for funding but are currently in final negotiations. Nor does it account for anticipated projects arising from upcoming competition of proposals submitted in response to new initiatives, such as the upcoming DoD Broad Agency Announcement (BAA) for 1999.
The scope of the federal research portfolio is broad. In size, projects range from small pilot studies to large-scale epidemiology studies and major research center programs utilizing significant amounts of appropriated research funds.
A table in Appendix A lists all of the research and development projects and programs supported now or in the past by the Federal Government. The appropriated funds centrally distributed to each program or project are shown in the fiscal years that funds were obligated.
Many extramural projects are multi-year efforts for which funds are obligated at the beginning of the project period. An entry of $0 is placed in the fiscal year column for each project for years in which funds were not obligated, but the project was ongoing. Blank entries for a project in any given fiscal year indicate a period of no research activity (years before a project was initiated, or years after a project was completed).
Table III-1 is a summary of research expenditures by DoD, VA, and HHS between FY’94 and FY’98, and a projection of funding into FY’99. Currently, the Federal Government is projecting cumulative expenditures of $133.5 million for research from FY’94 through FY’99. Through 1998, 40 projects have been completed, 103 projects are ongoing and 2 projects have been awarded funds and are pending start-up. Table III-2 is a year-by-year account of completed projects and new projects.
back to top
3. Diversity of Research Approaches
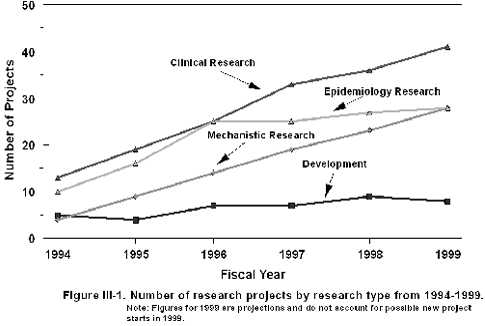
The funds that have been invested in research on Gulf War veterans’ illnesses over the years have gone into a broad-based portfolio with respect to research type and research focus area. Figure III-1 illustrates the number of projects of each research type for each year since FY’94. On the average epidemiology and clinical research have each comprised approximately about one third of the total number of projects. The remaining third has been divided between mechanistic research and development with the larger share going to mechanistic research. However, as the number of clinical and mechanistic research projects has increased over time, the number of epidemiology projects has become stable. This reflects a decreased need for additional studies of symptoms and illnesses as the ongoing studies begin to provide results.
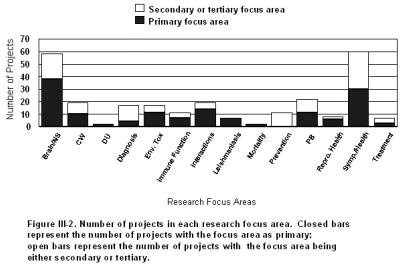
The distribution of projects across different research focus areas is illustrated in Figure III-2. Projects for each focus area are included based on whether the focus area is listed as one of the three focus areas assigned to each project. The total number of projects by research focus areas is shown in Figure III-2 in two ways. For each focus area, a black bar represents the total number of projects for which that focus area is listed as being primary. A clear bar represents the total number of projects for which the focus area is listed as being secondary or tertiary. Thus the total height of a bar represents the total number of projects for which the focus area is listed as primary, secondary, or tertiary. By showing the data this way the multiplicative effects of research investments are demonstrated. A project that examines Brain and Nervous System outcomes as a result of Pyridostigmine use is, therefore, counted under both of these focus areas.
As can be seen in Figure III-2, the overall emphasis of research has been greatest in the focus areas of the Brain and Nervous System and in Symptoms and General Health. This reflects the focus of epidemiological efforts on the prevalence of symptoms and illnesses in Gulf War veterans, and the focus of clinical research efforts on risk factors for illnesses. The focus on the brain and nervous system is a result of both the dominance of health complaints in this area, and the fact that many of the potential exposures in the Gulf are neurotoxins. The third largest investment in research is in the focus area of Diagnosis. This is a natural outcome of the research centered on characterizing Gulf War veterans’ illnesses, and on specific diagnostic tests such as the research on a serological assay for leishmaniasis.
The number of research projects in the various research focus areas has changed over time since 1994 as a reflection of the evolution of issues centered on Gulf War veterans’ illnesses. Of note is a relatively greater increase over the years of research on chemical interactions, chemical warfare agents and pyridostigmine bromide. These increases are an outgrowth of increased concern over the health risks posed to veterans by exposures to multiple toxic agents at low-levels.
back to top
4. Government and Non-Government Researchers
Research funded by the Federal Government is conducted at a variety of institutions and laboratories inside and outside of government. It has been important to the government to have a research portfolio that taps into the widest range of intellectual resources. Of the 145 projects that the government has sponsored over the years, less than half have been conducted at sites that almost exclusively involve government researchers. Many of these projects have taken advantage of resources unique to the Federal Government and would have been difficult to carry out by the non-government sector. An example includes the outstanding in-house expertise the Walter Reed Army Institute of Research has in the area of leishmaniasis research. Another is ready access to large military health and demographic databases. Non-government scientists, located primarily at universities, have been exclusively conducting approximately 30 of the projects. The remaining projects have involved government and non-government consortia of institutions and researchers. Examples include the VA Environmental Hazards Research Centers which entail close collaborations between VA medical center clinician/researchers and scientists at each VAMC’s affiliated university. Government/academic/private collaborations have been an invaluable asset to the research program because they combine the strengths of government and non-government laboratories and scientists. They also lead to outside ideas and approaches, and provide the government with objective and independent input into its research efforts.
back to top
IV.
NEW RESEARCH PROJECTS, INITIATIVES, AND MILESTONESBesides new research findings appearing in the scientific literature and at national meetings, there have been several important events since last year’s Annual Report to Congress for 1997 that deserve discussion. These include the awarding of new research projects and the development of new research initiatives including solicitations for new research.
Many of the new research projects and initiatives are the direct result of recommendations from a variety of sources including the Presidential Advisory Committee on Gulf War Veterans’ Illnesses (PAC, 1996a), the Institute of Medicine Panel on the Health Consequences of Service in the Persian Gulf War (IOM, 1996), and the RWG (PGVCB, 1995, 1996a)
back to top
1. Two New Treatment Protocols
As a result of the findings from epidemiological research to date, subgroups of ill Gulf War veterans have been identified for whom potential treatment trials are now appropriate, notwithstanding the lack of a comprehensive case definition for the symptom complex often called Gulf War Veterans’ Illnesses.
In the fall of 1997, the Research Working Group for Gulf War veterans’ illnesses approved a plan initiated by the VA and DoD to begin developing treatment trials for Gulf War veterans. In the spring of 1998, the VA Cooperative Studies Program initiated planning for two treatment trials, subsequently known as the ABT (antibiotic treatment) and EBT (exercise-behavioral therapy) trials. The planning effort for both trials took nine months. VA, in collaboration with the DoD, assembled scientists from the university community, private sector experts, and experts from the Federal Government, including the National Institutes of Health, DoD, and the VA itself. For both trials, experts from Europe were consulted during various aspects of the planning. As with all research approved by the Research Working Group, and in keeping with mandates from the Presidential Advisory Commission and the Congress, both trials underwent intense scientific review and scrutiny by the Cooperative Studies Evaluation Committee, a Federally Charted Research Review panel. Also, both trials were subjected to intense human studies review.
The four essential components of treatment trials include: 1) Strict criteria for entering the appropriate patients into the research study; 2) Provide treatments to patients with sufficient scientific rigor to determine definitively if the treatments work, and, if so, how well; 3) Systematic assessment of the treatment effect during active treatment and for a period of follow up after the treatment is stopped to see if an observed treatment effect is sustainable; and 4) Provide clear information to potential veteran research volunteers so they can make informed decisions regarding their participation in one of these trials.
Patient characteristics for entry into both treatment trials are similar. All Gulf War veterans who served in the Gulf between August 1990 and August 1991 are eligible for the studies. Inclusion criteria for entry requires that Gulf War veterans have at least two of the following three symptoms which began after August of 1990 and have lasted for more than six months continuing to the present. Symptoms targeted for treatment in the trials include: 1) Fatigue that limits usual activity, including work, recreation, or social activities; 2) Musculoskeletal pain involving two or more regions of the body; and 3) Neuro-cognitive symptoms, for example, difficulties in memory, concentration or attention. We expect that these symptoms will not likely be explained by any clearly defined disease condition, like arthritis, and no "Gulf War Syndrome" has yet been defined using credible scientific information. The antibiotic trial consists of some criteria that may lead to patients being excluded from the study, for example, patients with a special requirement for antibiotics for other conditions, or patients with allergies to tetracycline.
Exercise/Behavioral Therapy (EBT) Trial
The EBT trial focuses on the use of aerobic exercise and cognitive behavioral therapy in an effort to provide comprehensive, coherent and effective treatments to veterans and active duty soldiers ill after their service in the Gulf War. The primary study objective is to assess whether aerobic exercise combined with cognitive behavioral therapy (CBT) will improve the physical functioning of ill patients, and whether the combination of exercise and CBT will be more beneficial that either therapy alone. Additional study objectives include evaluation of any improvements in the cardinal symptoms of Gulf War Illnesses, especially pain, fatigue, and cognitive difficulties. We will also evaluate our ability to decrease level of disease related distress and improve emotional functioning of persons with Gulf War Illnesses.
One or more of these proposed treatment interventions are employed currently for patients ill with such conditions as Chronic Fatigue Syndrome, fibromyalgia, arthritis and back pain, headaches, and irritable bowel syndrome. The CBT intervention is not a singular approach to all the patient’s problems. Rather, it is a set of techniques that can be tailored for specific problems or illnesses. The techniques are used to produce cognitive change and are based largely on the development of problem solving skills. Unlike a drug, physical manipulation, or surgery where the patient is the passive recipient of a given treatment, CBT works best when the patients take an active role in the management of their symptoms. A growing body of research supports the idea that patient behavior and patient thinking can improve some illnesses to the same or greater degree than traditional medical approaches. CBT cannot "cure" Gulf War Veterans’ illnesses, but may help Gulf War veterans better manage their symptoms and improve their quality of life.
The EBT trial is taking place at 21 sites, most in VA but including two DoD sites. In order to answer the question of the benefit of these treatments, more than 1,350 patients will be studied. Patients are allocated to receive both aerobic exercise and CBT together, either treatment individually, or usual and customary care. Thus, there are four treatment groups. Patients are being enrolled over a one year period and will be followed for an additional year to assess the magnitude of the treatment effect and the durability of any observed benefit. The entire study will last about two years.
Antibiotic Treatment (ABT) Trial
The ABT trial focuses on a hypothesized infectious cause for the variety of symptoms experienced by Gulf War veterans. The putative organism is a species of Mycoplasma. Although there is no established, definitive link between infection with this organism and Gulf War veterans’ illnesses, undetermined numbers of ill veterans are taking the antibiotic Doxycycline for as long as one year in hopes of improving their health. In addition, there are anecdotal reports that this tetracycline has been useful in ameliorating the symptoms of Gulf War veterans.
A putative infectious etiology for Gulf War veterans’ illnesses provides an attractive hypothesis because antibiotics could possibly cure these patients’ symptoms. Unfortunately, anecdotal reports do not establish treatment benefit and a rigorous trial is necessary to address the question definitively. Accordingly, VA’s cooperative ABT trial’s primary hypotheses is to determine whether a 12-month course of antibiotic treatment (Doxycycline) directed against Mycoplasma species will improve the functional status of patients with Gulf War veterans’ illnesses who are tested as Mycoplasma positive. Note: to be able to enroll in this trial, patients who have the above symptom categories must also test positive for the presence of Mycoplasma genetic material. The study will also determine whether antibiotic treatment reduces symptoms including pain, fatigue, and neuro-cognitive difficulties, converts Mycoplasma positive patients to a Mycoplasma negative status, and whether the treatment benefit persists beyond one year.
The ABT is taking place at 30 sites, most in VA but including two DoD sites. Because this study will contain only two treatment groups, approximately 450 patients will be allocated to receive either antibiotic treatment or placebo. Patients are being enrolled over a one year period, treated actively for another year, then followed for an additional six months to assess the magnitude of the treatment effect and the durability of any observed benefit. The entire study will last about 2.5 years and is projected to cost approximately $12.4 million. The manufacturer of Doxycycline, Pfizer Pharmaceuticals, is donating the study drug and a matching placebo. The estimated size of this donation will exceed $5 million.
back to top
2. New Research Projects from DoD Broad Agency Announcement
In collaboration with the RWG, DoD developed a new research initiative for FY’98 (see Appendix C for the full solicitation). Under this initiative DoD solicited new research proposals for studies on the possible health risks associated with service in the Gulf War. The goals of this research are (1) to continue investigation of the pathogenesis of unexplained illnesses of Gulf War veterans; (2) to use the understanding of basic disease mechanisms to help ill veterans, and avoid or reduce the occurrence of such unexplained illnesses in future military deployments. This particular initiative was directed only at U.S. institutions of higher education (other than Federal Government) with degree-granting programs in science and/or engineering, or by consortia led by such institutions. DoD committed to spend up to $8 million in FY’98 for this initiative. The submission deadline was February 4, 1998.
The specific request focused on areas of research interest:
Submitted proposals in response to this BAA were scientifically peer reviewed by the American Institute of Biological Science. Highly meritorious proposals were referred to the Research Working Group of the Persian Gulf Veterans Coordinating Board for secondary review based on programmatic relevance, repetition of previous research, etc. This latter process is outlined in Section V. Research Management.
As a result of this process nine new DoD funded projects were initiated in FY’98/99. Table IV-1 lists each of these new projects providing brief summaries of the research approaches. In addition, DoD funded four other new projects (DoD-65-69) valued at over $7 million.
Table IV-1 New DoD Funded Projects for FY’98/99
| Project # | Title | Principal Investigator | Institution | Total Budget | Est. Comp. Date | Summary |
| DoD-65 | Multi-disciplinary pathophysiologic studies of neurotoxic Gulf War related syndromes leading to diagnosis and treatment | Robert Haley, M.D. | University of TX SW Medical Center at Dallas | $3,000,000 | 12/31/00 | Premised on the assumption that Gulf War Illness symptoms are due to nerve cell deterioration, the dual goals of this project are: to identify sites and pathophysiologic mechanisms of neurologic damage specific to the various symptoms and to use the acquired pathognomic information to set protocols for screening veterans for treatment. If successful the project could provide both means of identifying patients needing treatment and a basis for focused neurologic treatment. |
| DoD-66 | Testing for mycoplasmal infection replicability of nucleoprotein gene tracking and forensic polymerase chain reaction | Charles Engel, Jr., M.D. | WRAIR, WRAMC, Washington, D.C. | $263,825 | 11/30/99 | The goal of the project is to compare three tests used to detect mycoplasma fermentans, a parasitic microorganism of the genital mucosa which is difficult to culture, as a causative agent of suspected Gulf War related health problems. If successful, the project would provide a means of establishing differential diagnoses for specific symptoms in some Gulf War veterans. |
| DoD-67 | Antibacterial Treatment method based upon the excretion of dead and decaying spherical bacteria | Edward Hyman, M.D. | Louisiana Medical Foundation | $3,400,000 | 6/31/99 | The goal of the project is three-fold: (1) to identify the distinctive genotype of a suspected bacterial infectious agent in order to (2) establish and clarify a suspected link to symptoms in ailing Gulf War veterans, and (3) evaluate existing diagnostic and treatment methods for the agent for use with ill Gulf War veterans. If successful, the project would provide basic data on the infectious agent and clarify modes of infection and related symptomology useful both prophylacticly and in treatment. |
| DoD-69 | Five Year Follow-Up of Army Personnel Exposed to Chemical Warfare Agents | Dr. Phillip Renzullo | Institute of Medicine/Medical Follow-up Agency | $946,160 | 11/31/00 | The two goals of this project are to correlate illness and deaths of Gulf War veterans with possible exposure to chemical warfare agents at Khamisiyah, and to study the effect on an individual's health of self-concern of exposure to such agents whether or not actually exposed to them. Successful conclusion of the study may provide probabilities as to whether exposures to potentially harmful agents occurred and whether specific ailments in the Gulf War Illness constellation of symptoms correlate with that exposure site. The study may also provide information as to whether illness and death due to specific environmental exposures in Gulf War veterans can be reliably distinguished from ill health effects unrelated to specific site exposure. |
| DoD-70 | War Syndromes from 1900 to the Present: Symptom Patterns and Long-term Health Outcomes | Lt.Col Ian P. Palmer | Royal Defence Medical College, London | $734,687 | 12/31/00 | The goal of the project is to determine whether there are common elements in the health complaints or symptoms of veterans across several past wars. The project should provide information on types of health effects suffered by veterans due to involvement in war situations rather than due to specific etiologic exposures during a specific conflict. This information would aid in diagnosis of types of treatment necessary for veterans and in predicting interventions needed to reduce deleterious health effects post conflict. |
| DoD-71 | A Comparison of Post Deployment Hospitalization Between Vietnam and Gulf War Veterans | Christopher G. Blood | Naval Health Research Center, San Diego, CA | $566,000 | 12/31/99 | The goals of the project are to determine whether there are common health effects on veterans of two specific conflicts (Desert Storm and Vietnam), and to determine whether specific types of duty within the operations theatre correlate with post conflict health complaints in veterans. Information from the study may indicate the specific types of health complaints common to participation within a theatre of operations and distinguish types of complaints and risks of adverse health affects due to specific types of duty during a conflict. Such information could be useful in ameliorating adverse health consequences, in screening veterans likely to need treatment after a conflict, and provide insight into the types of treatments required. |
| DoD-72 | Long-term Effects of Subchronic Exposure to Sarin, Alone and with Stress or Other Chemicals | M. Abou-Donia, Ph.D. | Duke University Medical Center | $996,000 | 12/31/01 | The purpose of the project is to examine the effects of exposure to levels of sarin which normally are not associated with illness. The project studies will determine whether exposure, concurrently or sequentially, with other war-use chemicals or conditions (stress/heat) intensify the exposure consequences to low levels of sarin. The project will also explore the possibility that alteration in a natural neurologic protective barrier (the blood-brain-barrier) is a mechanism for possible increased toxicity of sarin exposure. Successful completion of the study will provide needed information on health effects of low level exposure to chemical agents, most specifically sarin, and to chemical interactions. |
| DoD-73 | Stress, Behavior and Health: Developing a Model for Predicting Post-deployment Morbidity, Mortality, and other Adverse Outcomes | Nicole Bell Sc.D., MPH | Social Sectors Development Strategies, Inc; Natick, MA | $500,000 | 12/31/01 | The goal of the project is to correlate (from information captured in health databases) the effect of individual preconflict attributes (e.g.behavior patterns, risk behavior, past background, etc..) and conflict and post-conflict experiences with ill health symptoms in veterans. The project may provide a means of predicting soldiers at greatest risk and may provide a means of identifying areas of concern in selecting troops for assignments and deployment. |
| DoD-74 | Relationship of Stress Exposures to Health in Gulf War Veterans | John A. Fairbank Ph.D. | Duke University Medical Center | $2,152,819 | 10/31/02 | The purpose of this project is: to examine and compare health problems of veterans who were either deployed or not deployed to the Gulf War theatre; and to correlate the health problems with variables comprising and mitigating stress situations experienced by veterans. The project may distinguish between health problems of deployed and non-deployed veterans and permit correlations with differentiating characteristics of stress situations and their impact on health. |
| DoD-75 | Toxic Interactions of Prophylactic Drugs and Pesticides | M. Abou-Donia, Ph.D. | Duke University Medical Center | $1,380,157 | 1/20/03 | The goal of this project is to clarify
interactive effects and mechanisms of combined exposure to pyridostigmine bromide, DEET
and permethrine. A second part will look at the integrity of the blood-brain-barrier
during application of pyridostigmine bromide, DEET and permethrine and combinations of
these chemicals. Successful completion of the project should provide information on how a
mixture of chemicals affects the body’s health status.
|
| DoD-76 | Evaluations of Immunotoxicity due to Concurrent Exposure to DEET, Pyridostigmine, and JP-8 Jet Fuel | Deborah Keil Ph.D. | Medical University of South Carolina, Charleston | $448,369 | 1/31/02 | This project will clarify the effect of varying single and concurrent exposures to pyridostigmine bromide, DEET and Jet fuel on neurotoxicity and, because of interdependencies of the immune and nervous systems, the resulting effect on immune function. The project may provide information on the potential effects of subchronic, low-level and concurrent exposures of the mentioned agents on specific immune functions and the consequence of associated immune alterations for adverse health effects. |
| DoD-77 | Percutaneous Absorption of Chemical Mixtures Relevant to the Gulf War | Jim E. Riviere D.V.M., Ph.D. | CCTRP, North Carolina State Univ.; Raleigh | $760,031 | 1/31/02 | The purpose of this project is to quantitate dermal absorption and cutaneous toxicity of chemical mixtures that veterans may have been exposed to during the Persian Gulf War and determine biomarkers associated with application of these mixtures. The project may provide information on chemical mixtures and their effects when applied dermally. It may also indicate specific biological alterations (biomarkers) useful in future research and diagnostic applications. |
| DoD-78 | Experimental Models of Gulf War Syndrome | Hugh Evans Ph.D. | New York University Medical Center | $2,179,097 | 1/31/03 | This project will develop experimental animal models for use in identifying causes and therapies for symptoms of Gulf War Syndrome that result from: inhalation of the organophosphate compounds soman or sarin, exposure to pyridostigmine bromide with its associated effects on cholinesterase activity, and psychological stress resulting from exposure to novel stimuli. Successful completion of the project should provide information on the effects of organophosphates delivered by inhalation (which is the route by which veterans may have been exposed) and permit estimation of whether toxic effects of the compounds are reversible. |
back to top
3. VA/DoD Neurobiology of Stress Initiative
The Final Report of the PAC (PAC, 1996b) recommended that additional research efforts be made in the area of stress and the neurobiology of stress. In 1997 VA and DoD issued a request
for proposals (see Appendix C4) from intramural VA and DoD clinician/scientists for research on stress and stress-related disorders. The neurobiological aspects of stress were emphasized in this solicitation. Proposals were submitted to VA by January 15, 1998. The Proposals underwent external scientific review by a joint VA/DoD appointed panel of experts, followed by programmatic review by the RWG. Nine new projects with a value of up to $5.0 million were funded in July 1998. Table IV-2 lists each of these projects with brief summaries of their research approaches.
Table IV-2 New VA/DoD Stress Funded Projects for FY’98/99
Project # |
Title |
Principal Investigator |
Institution |
Total Budget |
Est. Comp. Date |
Summary |
| DoD-79 | Time Course of Stress-induced Impairment of Blood Brain Barrier | G. J. Kant, Ph.D. | WRAIR, Washington, DC | $100,200 | 1/31/00 | The goal of this project is to determine whether prophylactic treatment for organophosphates with pyridostigmine bromide results in neurotoxicity under conditions of stress and intensifies the physiological or behavioral consequences of stress. Results of the project will provide information on pyridostigmine’s impact on cholinesterase blood and brain levels in animals under stress and will provide correlates of cholinesterase on stress-related hormones and cognitive performance. |
| DoD-80 | Molecular Regulation of Corticosteroid Receptor Expression in Stress-Responsive Cells | Jeffery Harmon, Ph.D. | USUHS, Bethesda, MD | $297,400 | 1/31/01 | The purpose of this project is to investigate specific genetic determinants (glucocorticoid receptor expression mechanisms) associated with dysfunction of the normal regulation of acute stress provided by the hypothalamic-adrenal-pituitary axis (HPA). Successful completion of the project may provide insight into the specific aberrations of the body’s regulatory mechanisms in stress situations. Knowledge of the specific aberrations should allow both a better understanding of somatization of stress-related disorders and tailored therapeutic interventions. |
| DoD-81 | Immunotoxicity due to Co-exposure to DEET, Pyridostigmine, and Stress | Gary Gilkeson, M.D. | VAMC, Charleston, N.C. | $300,000 | 12/31/01 | The goal of the project is to clarify, using an animal model, whether co-administration of DEET and pyridostigmine, in conjunction with and without physical stress, contributes to signs or symptoms of Gulf War Syndrome or other conditions by deleterious effects on the normal interaction of the nervous and immune systems. Results from this project may aid in determining the causal relationship between signs and symptoms of Gulf War veterans and exposure to DEET, pyridostigmine, and stress. |
| DoD-82 | Feasibility of Developing a Registry of PTSD Affected Veteran Sib Pairs | Seth Eisen, M.D. | VAMC St. Louis, MO | $172,000 | 9/30/00 | The goals of the study are to determine if sibling pairs at risk for PTSD are registered in an existing DoD health database. Results of the study would provide an investigational registry which could be used to explore linkage of genetic loci and PTSD. |
| DoD-83 | Risk for Stress-related Substance Abuse: the Effects of Family History of Alcoholism | Frances Gabbay, Ph.D. | USUHS, Bethesda, MD | $299,700 | 9/30/01 | The purpose of the project is to use the factors of family history which affect attentional processes and which existing studies link to substance and alcohol abuse for correlation with results of behavioral and electrophysiologic tests. Results of the project may permit construction of specific testing regimes to identify individuals at risk for substance abuse and who may be more sensitive to stresses related to military assignment. |
| DoD-84 | Psychobiologic Alterations in Persian Gulf War Veterans with and without PTSD | Julie Golier, Ph.D | VAMC Bronx, NY | $300,000 | 9/30/02 | The purpose of the project is to evaluate whether neuroendocrine profiles of Gulf War veterans with unexplained health problems can be correlated with similar profiles known to be associated with PTSD in Vietnam veterans and which are distinct from alterations seen in biologic responses to stress and other psychiatric disorders. The project will also attempt to determine whether there are correlations between profiles of neuroendocrine alterations and the health factor complaints described by Dr. Robert Haley. Results from the project may permit description of a common neuroendocrine profile useful in further research, diagnosis, and treatment of PTSD. |
| DoD-85 | CNS Cytokines and CRH in Gulf War Veterans with Multiple Unexplained Symptoms | Dewleen Baker M.D. | VAMC Cincinnati, OH | $299,100 | 9/30/02 | The first purpose of the project is to determine correlations between multiple unexplained Gulf War veteran health symptoms and concentrations of specific CNS cytokines and their neurohormonal modulators. The study will then assess the function of the hypothalamic-pituitary-axis in Gulf War veterans with unexplained physical symptoms compared to veterans without these symptoms and to a control group of individuals with a known stress related condition: fibromyalgia. Results of the project may provide diagnostic test strategies for specific veteran health complaints and indicate specific therapeutic interventions. |
| DoD-86 | Effects of Combat Stress on Structure and Function of the Hippocampus | Danny Kaloupek, Ph.D. and Steven Woodward, Ph.D. | VAMCs Boston & Palo Alto, CA | $597,600 | 9/30/01 | The purposes of the study are: to examine hippocampal volume in Gulf War veterans and Vietnam veterans with and without PTSD or alcohol abuse using enhanced-resolution magnetic resonance imaging and memory assessment; and to correlate the findings with existing studies which demonstrate reduced hippocampal volume in high-stressed groups. The project will also explore purported mechanisms of hippocampal volume reduction due to glucocorticoid mediated atrophy. Results of the study should provide a means of integrating findings from anatomic and biochemical measurements with correlated functional changes associated with hippocampal changes in Vietnam veterans with PTSD and Gulf War veterans with memory complaints. |
| DoD-87 | Measurement and Validation of Psychosocial Risk and Resilience Factors Accounting for Physical and Mental Health and Health-Related Quality of Life Among Persian Gulf War Veterans | Daniel King, Ph.D. | VAMC Boston | $289,100 | 9/30/01 | The goals of the project are to: provide an inventory of psychosocial risk and resilience factors (war-zone stress, predeployment and post war) for physical and mental health in military personnel, and to determine if the risk factors correlate with specific self-reported physical and mental health complaints of veterans. Results of the project may permit construction of an assessment inventory useful in future research, may assist identification of individuals at risk for adverse health effects, and may be an effective planning aid for prophylactic and treatment programs for future deployments. |
| DoD-88 | Clinical Relevance of Novel Immunological Markers in PTSD | Peter Morganelli, Ph.D. | VAMC White River Junction, VT | $242,300 | 1/10/01 | The purposes of the project are to study hypothalamic-pituitary-axis (HPA) effects on immune function by examination of expression of the glucocorticoid regulated glycoprotein p155 and other immune system parameters altered during a dexamethasone suppression (stress) test. Further, the project will correlate alterations in p155 expression and other parameters with specific symptoms and severity of PTSD. Results of the project may provide information on the relationship between HPA activity and immune function in PTSD and permit construction of diagnostic and management tests. |
| DoD-89 | Limbic Blood Flow and Opiate Receptor PET in Posttraumatic Stress Disorder | Lorraine Fig, M.D. | VAMC Ann Arbor, MI | $288,500 | 9/30/02 | The purposes of the project are to study hypothalamic-pituitary-axis (HPA) effects on immune function by examination of expression of the glucocorticoid regulated glycoprotein p155 and other immune system parameters altered during a dexamethasone suppression (stress) test. Further, the project will correlate alterations in p155 expression and other parameters with specific symptoms and severity of PTSD. Results of the project may provide information on the relationship between HPA activity and immune function in PTSD and permit construction of diagnostic and management tests. |
| DoD-90 | SPECT Benzodiazepine Receptor and MR Imaging in PTSD | Robert Innis, M.D. | VAMC West Haven, CT | $300,000 | 12/31/01 | The first goal of the project is to measure: hippocampal volume (involved in memory function), decreased density of hippocampal and frontal cortical BZ/GABA receptors (believed to play a role in anxiety), and memory in patients with PTSD. The second goal is to correlate findings with similar measures in PTSD patients from the Gulf War and Vietnam conflict. Results from the study may provide information on neurochemical and neuroanatomic correlates of PTSD, may provide information on the developmental time course of abnormalities (due to the difference in length of time since the Gulf and Vietnam conflicts), and may suggest diagnostic and therapeutic strategies for stress-related illness. |
| DoD-91 | Neurological and Circadian Substrates of PTSD-like Behaviors | Alan Frazer, Ph.D., Randy Strong, Ph.D., and Michael A. Rea, Ph.D. | VAMC San Antonio, TX/Armstrong Lab | $599,000 | 9/30/01 | The goal of the project is to use an animal model (with genetically based alterations in behavioral and physiologic responses to stress) to examine changes in circadian function, fear potentiated startle, and region-specific differences in biogenic amine systems to acute induced stress. Information provided by the project may provide a basis for further study of links between genetic susceptibility to stress-related disorders such as PTSD and alterations in specific brain systems (central catecholaminergic and serotonergic neuronal systems) and brain regions (amygdala, hippocampus, locus coeruleus, and suprachiasmatic). |
| DoD-92 | Traumatic Experiences Persistently Enhance Cue-dependent Learning: Toward an Animal Model of Chronic Stress and Posttraumatic Stress Disorder | Robert Servatius, Ph.D. | VAMC East Orange, N.J. | $249,700 | 9/30/02 | The goals of the study are: to determine whether induced, persistent neurobehavioral abnormalities, in an animal model, are correlated with persistent neuroendocrine changes; and whether conditioned behaviors affect startle sensitization. Successful completion of the study may clarify the effect of stressors in: altering learning processes, sustaining a chronic neuroendocrine stress state, and inducing and maintaining stress-related illness. |
back to top
4. Other New Initiatives and Research Related Initiatives
National Surveillance for Birth Defects Among Department of Defense (DoD) Health Care Beneficiaries
Building upon birth defect registry procedures established by the CDC and the California Birth Defects Monitoring Program, Naval Health Research Center (NHRC), San Diego recently concluded a feasibility study that screened 5,351 live births in San Diego County during the period 01/01/97 to 06/30/98. Six hundred fifteen individuals with birth defects were identified. The individuals were screened further for major birth defects of public importance, which yielded a prevalence of 3.2% major birth defects among 5,351 live births. This prevalence rate was consistent with birth defect data in the general population as reported by various states.
Upon conclusion of the feasibility study, NHRC proposed a Department of Defense (DOD) birth defects registry employing a hybrid case-finding approach that would include using passive methodology that screens existing DoD inpatient, outpatient, and Civilian Health and Medical Program of the Uniformed Services (CHAMPUS) data for infants meeting birth defect definitions. This passive surveillance would be supplemented with aggressive, active surveillance methods at Naval Medical Center San Diego (NMCSD), the largest DoD medical center. Active surveillance methods will include screening electronic discharge data and outpatient clinic logs for infants who fit the birth defect case definition. The data collection will use a standardized, computer-assisted abstraction program developed during the feasibility study. In order to verify the accuracy and completeness of the passive data, the information gathered during this active surveillance process at NMCSD will be matched to the passive data for the same catchment population and time periods. The percentage of agreement between the active and passive surveillance systems at this one site will be used to estimate the percentage of DoD birth defects collected using the passive system and to indicate if more aggressive data collection is needed. The active system will also permit comprehensive data collection for resultant epidemiological investigations. By using this hybrid approach, a high proportion of military personnel infants with birth defects will be detected. By not having to conduct active surveillance throughout the DoD, it will be possible to create a cost-effective birth defects registry.
The Assistant Secretary of Defense for Health Affairs established National Surveillance for Birth Defects among Department of Defense (DoD) Health Care Beneficiaries on November 17, 1998. The registry will meet the objectives of establishing surveillance, calculating semi-annual prevalence figures, and providing annual reports. As the registry grows, it will be possible to determine those birth defects that are most common, to calculate and to analyze rates for subgroups, and to create a data repository.
Depleted Uranium (DU)
Depleted Uranium was used by the U.S. military during the Gulf War in projectiles and armor for tanks. The medical effects of DU exposure are continuing to be evaluated. At a special DU clinical program at the Baltimore, MD, VA Medical Center, a group of Gulf War veterans with retained DU fragments from DU-contaminated wounds is being followed. The mission of this program was expanded in 1997 as other service members beyond those first identified by DoD in the 1993-94 timeframe, were also potentially exposed to DU, particularly through ingestion or inhalation. In coordination with VA Headquarters and the Baltimore VA program, the Office of the Special Assistant for Gulf War Illnesses (OSAGWI) initiated a program to assess potential DU exposure by job assignment or friendly fire involvement and to contact all soldiers to inform them of the possibility of exposure to DU and the availability of testing to determine current urine uranium levels. This approach to reviewing the toxicological and radiological effects of DU may improve the scientific basis for advice about fragment removal, to better understand uranium absorption in the body, distribution in human tissue, and how it is excreted. This may result in improved methods to assess uranium dose in humans. Thirty-three participants were evaluated between 1993 – 1997. While those individuals with evidence of retained shrapnel showed increased excretion of uranium, no association between uranium excretion and clinically detectable adverse health effects has been documented beyond those associated with their traumatic injuries. Efforts to improve both the assessment of uranium dose and the detection of toxic effects continue.
back to top
B. VA National Survey of Persian Gulf Veterans and their Family Members: Phase III
The purpose of the VA National Health Survey of Gulf War Era Veterans and Their Families (VA-2) is to estimate and compare the prevalence of various symptoms, medical conditions, and unexplained illnesses in Gulf War veterans and their family members, and those of non-Gulf War veterans and their family members. The National Survey is a population-based study being conducted in three phases.
In Phase I, a questionnaire was mailed to each of 30,000 veterans (15,000 Gulf War veterans; 15,000 non-Gulf War veterans) to obtain information on their exposures, presence of various symptoms, medical and psychological conditions, hospitalizations and reproductive health. Multiple follow-up mailings were made to increase the response rate. In Phase II, a telephone interview was attempted on all non-respondents using a CATI questionnaire, which includes a question on reasons for refusal. Telephone interviews with the non-respondents assist in assessing potential non-respondent bias and supplement the postal survey data. In addition, during Phase II, selected self-reported data collected by the postal questionnaire was validated through records review for 2,000 veterans from each group. Data collection from Phase I and II is complete with an overall response rate of 70% and data analysis is nearly complete.
Phase III is the most challenging part of the National Survey because it involves conducting medical evaluations on approximately 1,000 deployed and 1,000 non-deployed veterans, formed as a subset of participants from Phases I and II, and their immediate family members leading to medical evaluations of approximately 5,000 men, women and children. Because the National Survey itself is population-based, these veterans and family members must be drawn from across the nation thus creating potential logistical problems.
To design and operationalize Phase III, the VA Office of Research and Development (ORD) brought in the expertise of its Cooperative Studies Program. This program has a long history of designing and conducting complex multi-center drug and treatment efficacy trials. A Planning Committee for Phase III was headed by the Director of VA’s Cooperative Studies Coordinating Center at the Hines VA Medical Center in Chicago, Illinois. The other members of the Planning Committee came from VA National Headquarters and from VA Medical Centers around the country. These are researchers actively engaged in cooperative studies and epidemiology. The Planning Committee developed and submitted a comprehensive and detailed protocol to a subcommittee of the Cooperative Studies Evaluation Committee (CSEC, a federally chartered advisory committee) for an expedited mail review. The subcommittee approved the protocol for implementation in November 1997. During the six months following the protocol approval, data collection instruments and procedures were developed, study sites were selected, study personnel were hired and trained, and the data collection forms were submitted for requisite OMB approval. A letter of invitation to participate in the Phase III study was sent to the first group of 1181 veterans in June 1998.
Phase III clinical examinations began in September 1998 and are being carried out at 17 VA Medical Centers across the country. All VA Medical Centers that are participating have major university affiliations. The veterans are examined at the VA Medical Centers, while the spouses and children may have examinations performed under contract to affiliated university medical centers. The medical evaluations are designed to test the following a priori hypotheses:
Primary hypotheses:
Gulf War veterans will have an increased prevalence of the following medical and psychological conditions frequently reported in the literature compared to a control group of non-deployed era veterans:
Secondary Hypotheses:
Subject enrollment into Phase III is expected to take approximately 18 months. As of December 1998, 495 veterans agreed in writing to participate in the study.
C. New DoD Research Initiative
New funding for programmed research was established by the DoD. This program element funding is to address Gulf War Illnesses issues which may be of concern in future deployments, including issues identified in both the research plan of the PGVCB (PGVCB 1995, 1996a) and the Presidential Review Directive 5 (NSTC, 1998). This planned funding (approximately $19.5M per year for FY99 to FY02) makes it possible to organize multiyear research and improves the ability to respond to new research needs based on discoveries in the currently funded programs. The program is guided by an interservice panel and coordinated with the Research Working Group.
The overall objective of this effort is to enhance force health protection in future deployments through research specifically targeted to solving problems which emerged from service in the Gulf War. Specific research thrust areas include: (1) prevention and treatment of persistent stress symptoms, (2) methods to assess health hazards from toxic chemicals and mixtures and to monitor exposures, (3) improved safety assessments of medical materiel, including potential interactions in operational environments, (4) epidemiological studies to continue longterm followup of Gulf War veterans and to improve health status monitoring in future deployments, and (5) improved and accelerated research on Leishmaniasis prevention, diagnosis, and treatment.
Specific research solicitations will be used to develop focussed research efforts in
several of these areas, including interdisciplinary studies incorporating psychosocial and
neurobiology studies, development of toxicology hazard assessment methods and improved
understanding of military environmental interactions by researchers working closely with
DoD researchers, and new epidemiological studies pertaining to force health protection by
investigators with access to relevant populations and/or new approaches to data analysis.
The program will also extend the efforts of the Naval Health Research Center in San Diego
as the DoD Center for Deployment Health Research, and accelerate science and technology
efforts in the development of diagnostics, treatment drugs, and a vaccine for
Leishmaniasis in the DoD Military Infectious Disease Research Program.
back to top
D. Strategies for Future Deployment Health
1. National Science and Technology Council (NSTC)
As the PAC investigated the wide range of government health-related activities leading up to, during, and following the Gulf War, it identified a number of deficiencies in these activities that have contributed to current problems and controversies relating to Gulf War veterans’ illnesses. A major outcome of a recommendation contained in the Final Report of the Presidential Advisory Committee on Gulf War Veterans’ Illnesses has been the issuance of a Presidential Review Directive (PRD) instructing the National Science and Technology Council (NSTC) to develop an interagency plan to address future deployment health. Implementing this recommendation is significant in that it recognizes the importance of planning for the future while learning from the past.
PRD/NSTC-5 was issued in April 1997. Since that time an Interagency Working Group composed of four Task Forces (deployment health, research, records, and risk communication) developed strategic plans for their respective areas. These separate strategic plans were submitted to the Office of Science and Technology Policy of the Executive Office of the President in January 1998 for merging into one single strategic plan. A consolidated draft plan was submitted to the President’s Council of Advisors on Science and Technology (PCAST) for review and comment. A revised plan was then be submitted to NSTC in early spring, 1998, for final review and concurrence. On November 11, 1998 the final plan (NSTC, 1998) was released in conjunction with the announcement by President Clinton of the formation of the a new Military and Veterans Health Coordinating Board (MHVCB) (see Appendix B and D, respectively, for the Executive Summary of PRD-5 and the Charter of the MHVCB).
This strategic plan will provide a broad road map for all relevant departments to follow to ensure that the health of deployed servicemembers is optimal. Furthermore, it will enable the government to readily ascertain the nature and extent to which deployments affect the health of service members. The PRD/NSTC-5 can be accessed on the Internet at http://www.whitehouse.gov/WH/EOP/OSTP/NSTC/html/directive5.html.
back to top
2. National Academy of Sciences/Institute of Medicine
DoD has requested the advice of the IOM on a long-term strategy for protecting the health of our nation's military personnel when deployed to unfamiliar environments. The project will draw on the lessons of the Gulf War and subsequent deployments as well as a variety of other evidence to offer recommendations for: (1) an analytical framework for assessing the risks to deployed forces from a variety of medical, environmental, and battle-related hazards, including chemical and biological agents (CBA); (2) improved technology and methods for detection and tracking of exposures to these risks; (3) improved technology and methods for physical protection and decontamination, particularly of CBA; and (4) improved medical protection, health consequences management and treatment, and medical record keeping. The study began in October 1997 and will be completed by October 2000.
During the first year, principal investigators for each arm of the project have been selected and advisory panels of experts have been appointed. A large number of briefings, workshops, and site visits have been accomplished and an additional number of similar activities are planned for the second year. The purpose of these activities is to document current procedures, equipment, doctrine, and training as well as to review what is being developed or planned for the future. Draft outlines of the four reports due at the end of the second year are under review by the advisory panels. These reports will form the base for the final year’s activity - the production of a strategic plan for protecting the health of deployed forces in the future.
This strategic plan will serve as an important complement to the recently released NSTC-5 strategic plan (NSTC, 1998). Whereas the NSTC-5 plan will provide a broad road map for the government to follow, the NAS/IOM plan will provide a greater level of detail with specific recommendations for improvements, modernization, or efficiencies.
back to top
3. Military and Veterans Health Coordinating Board
President Clinton announced on November 11, 1998 the release of Presidential Review Directive/NSTC-5 (NSTC, 1998) that lays out an interagency plan for improving the Federal response to the health needs of our military, veterans and their families. In addition, the President took the first step in acting on this plan by directing the Secretaries of Defense, Health and Human Services, and Veterans Affairs to establish the Military and Veterans Health Coordinating Board (MVHCB) to oversee the plan’s implementation.
This document, along with the President’s directive, sets a course for improved cooperation and coordination among Federal agencies in addressing the health needs of our military, veterans, and their families. Through the release of this report and through the establishment of the MVHCB, the President is challenging the agencies to work together to achieve program synergy. What worked through the Persian Gulf Veterans Coordinating Board is being applied to future deployments. We expect continued coordination of research programs, record-keeping initiatives, deployment health efforts, and health risk communications.
The Coordinating Board will improve the level of coordination and communication between Federal agencies that have programs and expertise that can be brought to bear on the special needs associated with troop and veteran health. This has gone on in the past, but through the activities of the Board and its working groups this will become a more focused and effective process. Once established, the MVHCB would ensure coordination among VA, DoD, and DHHS on a broad range of health care and research issues relating to past, present, and future military service in the U.S. Armed Forces. The MVHCB is modeled on the Persian Gulf Veterans Coordinating Board, which is enhancinginteragency coordination, especially on research and clinical care related to health issues of Gulf War veterans.
The President established the Persian Gulf Veterans Coordinating Board in January 1994. The Secretaries of Defense, Veterans’ Affairs, and Health and Human Services are the official members. PGVCB has Clinical, Research, and Disabilities/Benefits working groups. PGVCB staff includes a VA Senior Medical Officer Executive Director, a DoD physician, a DoD research scientist, and an HHS administrator. The staff is located at VA Headquarters.
The PGVCB has provided a successful forum to address and coordinate DoD, VA, and DHHS activities regarding health care, benefits, and research programs for Gulf War veterans and Gulf War veterans' illnesses. DoD/VA meetings and DoD participation in interagency task forces responding to Presidential Review Directive/NSTC-5 (NSTC, 1998) further highlighted the importance of formally continuing interdepartmental collaboration.
The MVHCB does not replace the PGVCB. The staff of the PGVCB will support the MVHCB with the addition of one military officer to work deployment health issues. This arrangement will ensure a gradual transition to a board with a broader mandate and will highlight our long-term commitment to an interagency approach to future issues regarding the health of veterans and their families.
back to top
E. HHS Report to Congress on Multiple Chemical Exposures and Gulf War Veterans Illnesses
In response to House Report 105-205, in December 1997 HHS submitted to Congress a report (HHS, 1998) on its plans to develop a research program on the health effects of exposure to multiple chemicals. The report provided the Department’s initial plans.
The principal goal was to develop a five-year research plan to investigate the potential relationships between biological and chemical exposures in the Gulf War and subsequent illnesses in Gulf War veterans. One of the first activities was to convene a conference to provide a forum for broad public input into the development of a multi-year research plan for investigating the relationship between chemical exposures during the Gulf War and illnesses affecting Gulf War veterans. To accomplish this goal the Centers for Disease Control and Prevention (CDC), in coordination with the Office of Public Health and Science (Department of Health and Human Services), the National Institutes of Health, and the Agency for Toxic Substances and Disease Registry, sponsored a conference to address the health concerns of Gulf War veterans. This meeting, The Health Impact of Chemical Exposures During the Gulf War: A Research Planning Conference, took place on February 28 - March 2, 1999 in Atlanta, Georgia.
The format for the conference included plenary sessions and concurrent workgroups. The plenary sessions included a presentation on current research findings regarding the health impact of the Gulf War; a panel discussion of the experience of Gulf War veterans; a series of presentations on the possible health outcomes of low level chemical exposures focusing on nervous system, immune system, and pulmonary system outcomes; a series of panel discussions on research and clinical findings regarding multiple chemical sensitivity among Gulf War veterans and civilian populations; a series of presentations on possible mechanisms of action of chemical exposures; and a panel discussion on methodological considerations in studying the health impact of chemical exposures during the Gulf War.
Concurrent workgroups were held to develop research recommendations in the areas of pathophysiology/etiology of illnesses among Gulf War veterans; the most appropriate methods for assessing and diagnosing the health impact of chemical exposures; the most appropriate treatment approaches; and the prevention of similar illnesses in future military deployments.
In addition, there was a special Veterans Forum that served as an open forum for veterans to provide input regarding research priorities.
In addition to the CDC Conference, in FY’98 HHS augmented funding for an existing NIH Grant Announcement entitled "Chemical Mixtures in Environmental Health" (RFA: ES-98-002, NIH Guide, Volume 26, Number 38, November 21, 1997). This announcement was a joint effort of the National Institute of Environmental Health Sciences (NIEHS) and the Environmental Protection Agency (EPA). The announcement sought to fund research projects that would expand knowledge involving the characterization of "real-life" chemical mixture exposures based on human exposure or human body burden and to better understand the mechanisms of action involved as they relate to human health.
As a result of this initiative, NIEHS funded two new research projects. The first project is entitled Imunotoxicity of Dermal Permethrin and Cis-Urocanic Acid (HHS-7). The main objective is to examine immunotoxicity resulting from combined dermal exposure to permethrin and sunlight. The primary goal will be to provide new data to assist the estimation of risk from the combined immunotoxicant exposure. The second project is entitled Strategy to Identify Non-Additive Response to Chemical Mixtures (HHS-8). The overall goal of this project is to demonstrate a new strategy for identifying chemical mixtures that have non-additive biochemical response at relevant low exposures in order to eliminate the current strategies that depend on uncertain extrapolation of high dose, single compound data.
back to top
F. NAS/IOM Assessment of the Health Effects Associated with Exposures During the Gulf War
The ultimate value of the government’s investment in research on Gulf War veterans’ illnesses can only be realized when general conclusions can be drawn regarding such issues as the nature and cause(s) of the illnesses affecting Gulf War veterans. An assessment must encompass all research, whether directly related to the illnesses of Gulf War veterans, or indirectly related but highly relevant. Because of the important policy implications surrounding such an assessment, VA followed the advice of the PAC in its Special Report (PAC, 1997) to contract for the assessment with an independent scientific organization. VA contracted with NAS/IOM to carry out the assessment.
The project will be conducted in three phases. During the first phase, criteria will be developed for the identification of health outcomes of interest and for the selection of exposures to be examined. A review of the literature regarding some prototypic exposure-health effect associations will be conducted to develop methods to be used for analysis and synthesis of different types of research findings, e.g., animal toxicology data, occupational exposure data, and epidemiological data. In conducting reviews the committee will, for each medical condition considered, assess the latency periods, if any, between exposures to the potential risk factors and the manifestation of illnesses.
Scientific evidence concerning association of exposures and illness will be examined taking into account the strength of the scientific evidence and the appropriateness of the methods used to identify associations; whether the evidence indicates the levels of exposure of the studied populations were comparable to the exposures of Gulf War veterans, and whether there exists a plausible biological mechanism or other evidence of a causal relationship between exposure to the risk factor or factors and the medical conditions.
During phase two the remaining exposures will be subject to review and analysis. The final phase will be a series of updates of the literature and the associations, to be conducted every two or three years. An important aspect of this assessment is the development of research recommendations to fill identified gaps in knowledge.
back to top
G. Measuring the Health of Gulf War Veterans.
Longitudinal follow-up of the health status of Gulf War veterans is challenging, especially for ill-defined or undiagnosed conditions. VA and DoD have contracted with National Academy of Sciences/Institute of Medicine to develop a research design(s) and methods that could be used to measure the health of Gulf War veterans. The NAS/IOM established a committee to consider these methodological questions:
An IOM report was issued of a workshop held May 7, 1998 which reviewed completed population-based studies, VA and DoD data bases, descriptive statistics of Registry and CCEP participants, and a DoD draft protocol to evaluate the current well-being of CCEP participants. Information obtained is being used along with additional material and discussion to complete the task of designing measures for longitudinal comparison of the health status of Persian Gulf veterans to those who were not deployed to that conflict and methodologies to analyze longitudinal information in order to provide an accurate assessment of the health outcomes and treatment results in Gulf War veterans (IOM, 1998).
back to top
H. New DoD Center for Deployment HealthThe Defense Authorization Bill for Fiscal Year 1999 authorized the Secretary of Defense to establish a center devoted to "…longitudinal study to evaluate data on the health conditions of members of the Armed Forces upon their return from deployment…"
The Department shares with the President a firm commitment to "improve the health of our veterans, their families, and all who serve our nation, now and in the future." This responsibility includes minimizing adverse health effects that may be experienced during deployment. Critical to achieving this commitment will be establishment of Centers for the study of Deployment Related Health Concerns.
The goal of the DoD Centers is to ensure identification of trends in diseases, illnesses, or injuries among such members as a result of such operations.
Longitudinal studies to evaluate data on health conditions of members of the Armed Forces will include a wide range of clinical services, medical surveillance, and research activities. We envision Centers building on existing programs at Walter Reed Army Medical Center, the Army Center for Health Promotion and Preventive Medicine, and the Naval Health Research Center in San Diego. The Centers for Deployment Health will build and expand upon past Army, Navy, and Air Force experience. The goal of the Centers will be to improve our ability to prevent, investigate, and ameliorate future outbreaks of unexplained illness after deployments. The Comprehensive Clinical Evaluation Program and Specialized Care Program for Gulf War Veterans will be programs within the proposed Clinical Center.
The proposed mission of the Centers for Deployment Health would focus on four primary areas: force health protection, surveillance, clinical care, and research.
This mission would require collaboration with multiple agencies, including the Departments of Veterans Affairs and Health and Human Services. The inter-agency collaborative effort will be managed through the Military and Veterans Health Coordinating Board.
back to top
I. VA Center for War-Related Illnesses
Section 103 of P. L. 105-368 requires VA to enter into an agreement with NAS/IOM to help VA develop a plan for the establishment of a "VA National Center on War-Related Illnesses and Post-Deployment Health Issues." On December 18, 1998, the IOM's Division of Health Promotion and Disease Prevention submitted a proposal to VA (No. 99-IOM-127) for a study of such a plan. The IOM study will assess preliminary VA plans and make recommendations regarding such efforts. Elements of the proposal include the establishment of an expert committee, a planning workshop, and two additional committee meetings. The expert committee will provide VA with a final report detailing its recommendations on the feasibility and possible structure and mission of the proposed National Center. VA strongly supported this legislative proposal because a National Center will enhance our ability to care for post-conflict veterans and extend VA's research capabilities in this area.
back to top
J. Gulf War Veterans’ Illnesses Research Advisory Committee
Section 104 of Public Law 105-368 directs the Secretary of VA, as the designated Executive Agent for the coordination of all Gulf War veterans’ illnesses research activities funded by the Executive Branch of the Federal Government, to establish a public advisory committee on Gulf War veterans’ illnesses research. Specifically, the purpose of the committee will be to provide advice and make recommendations to the Chairperson of the RWG (VA’s Chief Research and Development Officer) and other appropriate entities including Gulf War veterans, on the nature, scope, and direction of federal research activities overseen and coordinated by the RWG.
The committee will be called the Research Advisory Committee on Gulf War Veterans’ Illnesses (RACGWVI). The purview of the Committee will include the policies and programs overseen and coordinated by the RWG with respect to research on Gulf War veterans’ illnesses conducted or sponsored by any agency or department of the Executive Branch of the Federal Government.
As a part of its function, the Committee may accept suggestions for research and development from Congress, VA and non-VA scientists, veterans and their representatives, and the general public.
Membership on the Committee will include, but not necessarily limited to: (i) Gulf War veterans and/or their representatives; (ii) basic biomedical researchers; (iii) epidemiological researchers; (iv) clinical researchers; (v) environmental and occupational health researchers; and (vi) mental health and behavioral researchers. Close attention will be given to equitable geographic distribution and to ethnic and gender representation.
The federal charter for the Committee is currently under review, and nominations for membership are being solicited. It is expected that the first Committee meeting will occur in the last quarter of FY’99.
back to top
K. 1998 Medical Defense Bioscience Review: Low-Level Chemical Agent Research
The Department of Defense sponsored and conducted its 1998 Medical Defense Bioscience Review in Hunt Valley, Maryland May 31 – June 4, 1998. The overall topic of the 1998 Medical Defense Bioscience Review was low-level chemical agent research. Selected papers from that meeting were published in the Journal Drug and Chemical Toxicology. Specific topics ranged from the toxicokinetics of sulfur mustard to the neuromuscular effects of low-level exposures to sarin, PB, DEET, and chlorpyrifos. One paper (Moore, 1998) was a comprehensive review of existing data on the health effects of exposure to low-levels of nerve agents. The author concludes that at the present time controlled studies of human exposures, reports of accidental exposures, and animal studies collectively indicate that exposures to organophosphorous nerve agents at levels causing no acute signs or symptoms do not produce chronic illness.
back to top
L. 1998 Conference on Federally Sponsored Gulf War Veterans’ Illnesses Research
The RWG organized and hosted an international meeting, "Conference on Federally Sponsored Gulf War Veterans’ Illnesses Research" June 17-19, 1998 in Crystal City, VA. The purpose of the meeting was to bring federally sponsored researchers on Gulf War veterans’ illnesses together in a common forum to:
Over 240 participants registered for the Conference that involved plenary sessions with invited speakers, and oral platform and poster presentations from scientists who submitted abstracts of their research findings to the Conference Technical Planning Committee. There were 16 invited plenary speakers from government and university institutions discussing a range of topics from establishing case definitions to the future of force health protection. Nine breakout sessions featured oral presentations by 44 speakers who had submitted abstracts. In addition the poster session featured 45 posters on a range of research topics.
Besides the research sessions, there were a number of clinical sessions targeted more at clinicians treating Gulf War veterans’ illnesses.
In 1999, the RWG will host another Conference on Federally Sponsored Gulf War Veterans’ Illnesses Research June 23-25, 1999 in Crystal City, VA. The overall purpose and goals of the meeting will be similar to the 1998 meeting, but there will be a greater emphasis on translating research into clinical practice. In addition, although the Conference is targeted toward federally sponsored research, the meeting will be open to the general public on a space-available basis. More information on these meetings is available in Appendices E and F.
back to top
V. RESEARCH MANAGEMENT
Research on Gulf War veterans’ illnesses is a complex undertaking, involving a number of different approaches. The federal research effort on this problem involves scientists in federal, academic, and private institutions, both in the United States and abroad, conducting research sponsored by VA, DoD, and HHS. Each of these Departments has distinct, though complementary, capabilities and capacities for conducting and sponsoring research on Gulf War veterans’ health issues. In addition, each Department has its own appropriation for extramural and intramural general biomedical research programs.
The biomedical research programs in VA, DoD, and HHS have well established management structures for science policy formulation and the solicitation, scientific peer review and funding of both extramural and intramural programs. Each Department’s research management hierarchy for Gulf War veterans’ illnesses research has been linked through an overall policy and management coordination effort carried out by the Research Working Group (RWG) of the Persian Gulf Veteran’s Coordinating Board (PGVCB). As an operational policy, RWG works through the line management authority each department maintains over its intramural scientists, scientific program managers (responsible for extramural research), and their budgets. The RWG has no budget authority itself, however. As a consequence, all funds for research flow through the funding agency or department.
Each Department engaged in research on Gulf War veterans’ illnesses endorses the need for both prospective and retrospective scientific peer review of research. Because of the urgency of the health concerns of Gulf War veterans and their families, and the diverse nature of the reported illnesses, review and oversight of research have been important. VA, DoD, HHS, and the Executive Office of the President have established multiple oversight mechanisms to capture the full spectrum of the overall effort; some oversight mechanisms are broad-based, encompassing all research issues, whereas others are more focused on individual research projects and programs.
Four of the most important oversight activities are briefly discussed below. Although each has had a broad mandate encompassing virtually all issues related to Gulf War veterans’ illnesses, the discussion below will focus on their research oversight activities, findings, and recommendations.
back to top
As directed by P.L. 102-585 Section 706, in 1993 VA and DoD jointly entered into an initial 3 year contract with the Medical Follow-Up Agency (MFUA) of the Institute of Medicine, National Academy of Sciences. The IOM was charged with reviewing existing scientific, medical and other information on the health consequences of military service in the Persian Gulf area during the Gulf War. The IOM was also mandated to review the research activities and plans of the various involved agencies and make recommendations.
On January 4, 1995, the IOM issued a report entitled "Health Consequences of Service During the Persian Gulf War: Initial Findings and Recommendations for Action" (IOM, 1995). In general the IOM panel endorsed previous findings of the Defense Science Board (DSB, 1994) and a National Institutes of Health Technology Assessment Conference (NIH, 1994) that no single disease entity with no single etiology could be identified for the health complaints expressed by Gulf War veterans. The panel also strongly emphasized the importance of population-based studies, which are currently ongoing in a number of areas. At that time the panel found no evidence that either chemical or biological warfare (CBW) agents were used against coalition troops during the Gulf War and, as a consequence, recommended that this not be a factor in future considerations of the causes of Gulf War veterans’ illnesses.
In the final IOM report of September 1996 (which stands as a separate document from its initial January 1995 report) the IOM panel stated that it could find no scientific evidence to date demonstrating adverse health consequences associated with service in the Gulf War beyond the few documented cases of leishmaniasis, combat-related or injury-related mortality or morbidity, and increased risk of psychiatric sequelae of deployment. The panel went on to state that there is a strong likelihood that no single hypothesis could account for all illnesses reported by Gulf War veterans, whether or not they resulted from service in the Gulf War. Finally, the panel observed that after previous wars and conflicts, a proportion of military service personnel and veterans have had medical complaints of varying degrees of severity that are not explainable based on identifiable health hazards or physical illnesses. This observation echoes work by Hyams et al. (1996) tracing such a phenomenon back at least to the Civil War.
The IOM panel made the following research recommendations:
As authorized by P.L. 102-585 sec. 706, the agreement with IOM has been extended for the general purposes of providing core epidemiological support for research on military and veteran populations. This support is a valuable infrastructural base to carry out epidemiological research on Gulf War veterans’ illnesses.
back to top
2. Research Advisory Committee on Gulf War Veterans’ Illnesses
As discussed in Section IV-J above, section 104 of Public Law 105-368 directs the Secretary of VA, as the designated Executive Agent for the coordination of research on Gulf War veterans’ illnesses funded by the Executive Branch of the Federal Government, to establish a public advisory committee on Gulf War veterans’ illnesses research. Specifically, the purpose of the committee will be to provide advice and make recommendations to the Chairperson of the RWG (VA’s Chief Research and Development Officer) and other appropriate entities including Gulf War veterans, on the nature, scope, and direction of federal research activities overseen and coordinated by the RWG.
The committee will be called the Research Advisory Committee on Gulf War Veterans’ Illnesses (RACGWVI). The purview of the Committee will include the policies and programs overseen and coordinated by the RWG with respect to research on Gulf War veterans’ illnesses conducted or sponsored by any agency or department of the Executive Branch of the Federal Government.
The federal charter for the Committee is currently under review, and nominations for membership are being solicited. It is expected that the first Committee meeting will occur in the last quarter of FY’99.
back to top
The President established the PAC by Executive Order on May 26, 1995. Between August 1995 and January 1997 the committee met a total of 23 times either as a full committee or in subcommittees. The 12 member committee was composed of scientists, health care professionals, veterans, and policy experts. The Committee was charged with reviewing and providing recommendations on the full range of activities relating to the government’s response to Gulf War veterans’ illnesses. In addition the Committee evaluated the available data on the nature of Gulf War veterans’ illnesses and on potential health effects related to Gulf War risk factors.
The Committee released an interim report in February 1996 (PAC, 1996b). Although the Interim Report stated that VA, DoD, and HHS research programs are generally well-designed and should lead to meaningful answers to issues concerning Gulf War veterans-related health issues, it also had several recommendations. The Committee’s recommendations covered issues such as peer review, coordination of agency research activities, the use of public advisory committees and the availability of information on troop exposures. The Committee made no findings about specific illnesses or risk factors in the Interim Report. In response to the Interim Report, the agencies developed a coordinated plan of action (PGVCB, 1996b) that responded to the Advisory Committee’s interim recommendations.
The Final Report of the Committee was released in December 1996 (PAC, 1996a). The PAC came to the following conclusions:
Pesticides
Chemical warfare agents
Biological warfare agents
Vaccines
Pyridostigmine bromide
Infectious diseases
Depleted uranium
Oil well fires and smoke
Petroleum products
The Final Report also concluded that stress (known to affect the brain, immune system, cardiovascular system, and various hormonal responses) is likely to be an important contributing factor to Gulf War veterans’ illnesses.
The Final Report made the following research recommendations:
Because of concern over the adequacy of DoD investigations into reports of possible chemical and biological warfare exposure incidents during the Gulf War, and because of a need to follow up on recommendations the Committee’s Final Report, the President extended the Committee through October 1997. At that time the PAC issued a Special Report (PAC, 1997). In the Special Report the Committee did not alter its findings and conclusions with respect to potential causes of Gulf War veterans’ illnesses. The Special Report did not contain any specific recommendations for research.
With respect to federally funded research on Gulf War veterans' illnesses, the PAC concluded that the government has been adequately and appropriately responding to its recommendations. The PAC particularly commended the government for its new initiatives targeted on health effects of low-level exposure to Chemical Warfare (CW) agents. As the PAC noted in its Final Report, "the amount of data from either human or animal research on low-level exposures [to CW agents] is minimal." However, in its Special Report, the PAC concluded that planned research on the health effects of low-level exposure may address any uncertainties and inconclusiveness identified in the Final Report.
The PAC approved of the government's targeted solicitations for research and the process used to make such awards. However, the Committee expressed reservations about a perceived degradation in DoD's processes for funding research related to Gulf War veterans' illnesses. The Committee noted that "competition and external peer review of research proposals are essential to guarantee scientific merit, relevance, and level of priority generally." The Committee acknowledged that benefit can accrue from small-scale, short-term funding on a sole source basis for pilot projects or to address narrow scientific questions (e.g., DoD's recent funding of $100,000 for technical issues related to a test for possible Mycoplasma infection). The Committee stated, though, that such approaches should be rare and that protocols still should be peer reviewed prior to funding, limited in the amount of funds released, and not subject to renewal without competition.
The RWG of the PGVCB will continue to endorse peer review and competition as the means of obtaining the best research products.
back to top
In addition to the broad oversight that has been provided by the three committees cited above, there are several standing and special committees responsible for oversight on individual research projects and programs. Projects and programs receiving continuous or ad hoc oversight include:
The RWG also continues to encourage that all large epidemiological studies establish public advisory groups that include representation of Gulf War veterans.
Additionally, valuable review and oversight has been provided by the Senate Veterans Affairs Committee Special Investigations Unit, the House Committee on Veterans’ Affairs, the House Government and Reform Oversight Committee, the Presidential Special Oversight Board, and the General Accounting Office.
back to top
In 1993, VA, DoD, and HHS recognized the importance of a coordinated approach to research on Gulf War veterans’ illnesses and formed the "Persian Gulf Interagency Research Coordinating Council". By January 1994, when the Secretaries of VA, DoD, and HHS formed the Persian Gulf Veterans Coordinating Board,
the Research Coordinating Council became the Research Working Group operating under the auspices of the Coordinating Board (Beach, et al., 1995). Because of the potential link between environmental factors and Gulf War veterans’ illnesses, the Environmental Protection Agency was asked to be a member of the Research Working Group. Figure V-1 shows the structure
of the Research Working Group. Figure V-1 includes various subcommittees that have been in existence over the past several years. The Planning Subcommittee for Annual Meetings of Federal Researchers has been responsible for organizing three meetings of federally funded researchers, and is currently planning a fourth. These meetings provide an opportunity for researchers to gather together to share recent research results and to discuss research problems of mutual interest. The June ‘99 conference will be an open meeting allowing for extensive interactions among researchers, veterans, and other members of the public.
The RWG is charged with assessing the state and direction of research, identifying gaps in factual knowledge and conceptual understanding, identifying testable hypotheses, identifying potential research approaches,
reviewing research concepts as they are developed, collecting and disseminating scientifically peer-reviewed research information, and insuring that appropriate peer review and oversight are applied to research conducted and sponsored by the Federal Government. Membership on the Research Working Group consists of senior research scientists and clinical managers from VA, DoD, HHS, and EPA.
Programmatic Review of Extramural Research Proposals
An important function of the RWG is programmatic review and recommendation to
funding agencies of research proposals that have been competitively peer reviewed. Figure V-2 illustrates the general approach the RWG has taken to extramural research (research funded by an agency, but carried out by organizations outside of the agency such as universities, private laboratories, or other independent government agencies). The RWG works collectively with VA, DoD, and HHS to establish research needs and identify agency-specific funding mechanisms to support that research. For a specific research funding activity, the responsible funding agency works in coordination with the RWG to develop a targeted solicitation for research. Proposals that are submitted to the funding agency in response to a solicitation are scientifically peer-reviewed using agency-specific peer review programs (e.g., DoD/Department of the Army (DA) uses a contract with the American Institute of Biological Sciences). Abstracts of peer-reviewed proposals, written reviews of the peer-reviewers, and the scientific merit scores assigned by the peer-reviewers, are provided to a subcommittee of the RWG charged with
providing secondary review of proposals for relevance. This material is redacted for personal and institutional identifiers. Relevance determinations are guided by programmatic needs articulated through the RWG process and reflected in the Working Plan. In its secondary
review the RWG may re-rank proposals based on relevance, but it will not recommend funding any non-meritorious proposal, irrespective of relevance or funds availability, to any agency.
Though scientists within intramural research programs do not compete for their funding in the same way as non-federal extramural scientists, the RWG works with agencies to ensure that intramural programs and projects are adequately peer-reviewed.
The RWG will continue to work diligently to foster the highest standards of competition and peer-review for all research on Gulf War veterans’ illnesses.
Notable among the activities of the RWG are:
back to top
VI. RESEARCH PRIORITIES
During 1998, the Research Working Group, as a part of its regular functions, undertook the matter of research priorities for FY’99 and beyond. Key factors that guided the RWG in it discussions were: recent research findings; the current breadth and depth of the research portfolio in key areas; and the availability of resources to develop needed new initiatives.
A. Research on Treatments for Gulf War Veterans’ Illnesses
With respect to research findings, the RWG continues to take note of the growing population-based epidemiological evidence, including very recent publications by the CDC (Fukuda, et al. 1998) and by researchers from Great Britain (Unwin, et al. 1999), suggesting that Gulf War veterans with unexplained illnesses are suffering from a complex of symptoms such as fatigue, musculoskeletal pain, and cognitive problems. Furthermore, such studies, along with smaller clinical studies, have been unable to define a specific syndrome, or assign a specific cause for these symptom complexes. The RWG has noted that these symptom complexes significantly overlap with other symptom complexes identified in the civilian population such as Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). As with Gulf War veterans’ illnesses, no clearly defined etiologic agent has been identified for CFS and FM. With these observations in mind the RWG felt that experimental treatments methods that have been applied to persons with CFS or FM deserve further exploration in the context of Gulf War veterans’ illnesses. Consequently, the RWG established the development of treatment protocols for unexplained illnesses as a research priority for future years.
back to top
B. Longitudinal Follow-Up for Gulf War Veterans’ Illnesses
Although several individual research projects funded by the Federal Government have longitudinal components built into them, no research is specifically directed toward understanding the progress of Gulf War veterans’ illnesses over time. The RWG has concluded that to the extent feasible, research approaches need to be applied to determine the long-term health of Gulf War veterans in contrast to the several cross-sectional epidemiological research projects recently completed or still ongoing.
back to top
The approximately 10% of veterans who have reported ill health following deployment to the Gulf War represents a significant level of morbidity that might be preventable. In concert with the growing need to better understand the health problems of Gulf War veterans so that future health problems in future deployments can be prevented, the RWG has endorsed future research aimed generally at disease prevention, and more specifically at prevention of stress-related symptoms and conditions. As a result of RWG recommendations in the past, the federal investment in the pathophysiology of stress-related illnesses has markedly increased. With this investment in better understanding the pathophysiology stress-related illnesses, it is also appropriate to initiate research that can take advantage of new findings in that area and how they might lead to better disease prevention, and even treatment.
back to top
As has been recognized in Presidential Review Directive (PRD; NSTC, 1998), the ability to better anticipate environmental and occupational hazards prior to deployments could potentially reduce morbidity, and even mortality, associated with unintended or unanticipated exposures. The RWG, in conjunction with the Working Groups that developed PRD/NSTC-5, recommend enhanced research efforts aimed at improving methods of hazard identification and risk assessment for environmental and occupational hazards. Such research efforts should emphasize the reality of complex multiple exposures to more than one hazardous agent.
back to top
With respect to treatment of Gulf War veterans’ illnesses, in 1998 VA and DoD designed two major multi-site treatment trials. Protocols for these two trials were approved by outside peer review in late 1998 and funded to begin recruiting subjects in Spring 1998. Details of those two trials are provided in Section IV.A of this Report. Total investment by VA and DoD for these two trials are likely to be in excess of $15 million and $5 million, respectively.
Also, in 1998 the DoD established new funding for programmed research. This "program element" funding, explicitly put into DoD’s budget requests, is to address Gulf War Veterans’ Illnesses issues which may also be of concern in future deployments, including issues identified in both the research plan of the PGVCB (PGVCB, 1995 and 1996a) and the Presidential Review Directive 5 (NSTC-5).
The overall objective of this effort is to enhance force health protection in future deployments through research specifically targeted to solving problems which emerged from service in the Gulf War. Specific research thrust areas include: (1) prevention and treatment of persistent stress symptoms, (2) methods to assess health hazards from toxic chemicals and mixtures and to monitor exposures, (3) improved safety assessments of medical materiel, including potential interactions in operational environments, (4) epidemiological studies to continue long-term follow-up of Gulf War veterans and to improve health status monitoring in future deployments, and (5) improved and accelerated research on Leishmania prevention, diagnosis, and treatment.
More information on the DoD initiative can be found in Section IV.C of this Report.
back to top
VII. REFERENCES
Beach P, Blanck RR, Gerrity TR, Hyams KG, Mather S, Mazzuchi JF, Murphy F, Roswell R, Sphar RL. Persian Gulf Veterans Coordinating Board: organization, mission, accomplishments. Fed Prac, December 9-15, 1995.
Coker WJ, Bhatt BM, Clatchley NF, Graham JT. Clinical findings for the first 1000 Gulf War veterans in the Ministry of Defence’s medical assessment programme. British Medical Journal, 318:290-4, 1999.
DSB (Defense Science Board). Final Report: Defense Science Board Task Force on Persian Gulf War Health Effects. Washington, D.C.: Office of the Under Secretary of Defense for Acquisition and Technology, 1994.
Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcription response. Nature Medicine, 2:1382-5, 1996.
Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaskt BL, Mawle AC, Reeves WC. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA, 280(11):981-8, 1998.
Gray GC, Coate BD, Anderson CM, Kang HK, Berg SW, Wignall FS, Knoke JD, Barrett-Connor E. The postwar hospitalization experience of US Persian Gulf War veterans. N Engl J Med, 335:1505-13, 1996.
Gray GC, Hawksworth AW, Smith TC, Kang HK, Knoke JD, Gackstetter GD. Gulf veterans’ health registries. Who is likely to seek evaluation? Amer J of Epidemiol, 148:343-349, 1998.
(HHS) Department of Health and Human Services. Report to Congress: Research on Multiple Chemical Exposures and Veterans with Gulf War Illnesses, January 1998.
Hyams KC, Wignall FS, Roswell R. War syndromes and their evaluation: from the U.S. Civil War to the Persian Gulf War. Ann Int Med; 125(5):398-405, 1996.
IOM (Institute of Medicine). Health Consequences of Service During the Persian Gulf War: Initial Findings and Recommendations for Immediate Action. Committee to Review the Health Consequences of Service During the Persian Gulf War. Medical Follow-up Agency; Institute of Medicine. National Academy Press, Washington, DC, 1995.
IOM (Institute of Medicine). Health Consequences of Service During the Persian Gulf War: Final Report. Committee to Review the Health Consequences of Service During the Persian Gulf War. Medical Follow-up Agency; Institute of Medicine. National Academy Press, Washington, DC, 1996.
IOM (Institute of Medicine). Measuring the Health of Persian Gulf Veterans: Workshop Summary. Committee on Measuring the Health of Persian Gulf Veterans. National Academy Press, Washington, DC, 1998. http://books.nap.edu/catalog/6260.html.
Ismail K, Everitt B, Blatchley N, Hull L, Unwin C, David A, Wessely S. Is There A Gulf War Syndrome? Lancet, 353:179-82, 1999.
Ismail K, Everitt B, Blatchley N, Hull
Knoke JD. Gray GC. Hospitalizations for unexplained illnesses among U.S. veterans of the Persian Gulf War. Emerg Infect Dis, 4(2):211-9, April-June 1998.
Knoke JD, Gray GC, Garland FC. Testicular cancer and Persian Gulf War service. Epidemiology, 9(6):648-53, November 1998.
Lallement G, Foquin A, Baubichon d, Burkhart MF, Capentier P, Canini F. Heat stress, even extreme, does not induce penetration of pyridostigmine into the into the brains of guinea pigs. Neurotox, 19(6):759-66, 1998.
Marino MT, Schuster BG, Brueckner RP, Lin E, Kamainskis A, Lasseter R. Population pharmacokinetics and pharmacodynamics of pyridostigmine bromide for prophylaxis against nerve agents in humans. J Clin Pharmacol, 38(3):227-35, 1998.
Moore DH. Health Effects of exposure to low doses of nerve agent – a review of present knowledge. Drug and Chemical Toxicology, 21(1)123-130, 1998.
NIH (National Institutes of Health Technology Assessment Workshop Panel). The Persian Gulf experience and Health. JAMA; 272(5):391-396, 1994.
NSTC (National Science & Technology Council). Presidential Review Directive 5. 1998.
PAC (Presidential Advisory Committee on Gulf War Veterans’ Illnesses). Final Report, 1996a.
PAC (Presidential Advisory Committee on Gulf War Veterans’ Illnesses). Interim Report, 1996b.
PAC (Presidential Advisory Committee on Gulf War Veterans’ Illnesses). Special Report, 1997.
Pollet C, Natelson BH, Lange G, Tiersky L, DeLuca J, Policastro T, Desai P, Ottenweller J, Korn L, Fiedler N, Kipen H. Medical evaluation of Persian Gulf veterans with fatigue and/or chemical sensitivity. Journal of Medicine, 29(3&4):101-13, 1998.
Proctor SP, Heeren T, White RF, Wolfe J, Borgos MS, Davis JD, Pepper L, Clapp R, Sutker PB, Vasterling JJ, Ozonoff D. Health status of Persian Gulf War veterans: self-reported symptoms, environmental exposures and the effect of stress. Int J Epidemiol, 27:1000-10, 1998.
PGVCB (Persian Gulf Veterans Coordinating Board). A Working Plan for Research on Persian Gulf Veterans Illnesses, August 5, 1995.
PGVCB (Persian Gulf Veterans Coordinating Board). A Working Plan for Research on Persian Gulf Veterans’ Illnesses, 1996a.
PGVCB (Persian Gulf Veterans Coordinating Board). Action Plan with Respect to the Findings and Recommendations of the PAC on Gulf War Veterans’ Illnesses, 1996b.
Servatius RJ, Ottenweller JE, Beldowicz D, Guo W, Zhu G, Natelson BH. Persistently exaggerated startle responses in rats treated with pyridostigmine bromide. J Pharmacol Exp Therapeutics, 2871020-8, 1998.
Stuart JA, Bliese PD. The long-term effects of Operation Desert Storm on the Psychological Distress of US Army Reserve and National Guard veterans. Journal of Applied Social Psychology, 28:1-22, 1998.
Unwin C, Blatchley N, Coker W, Ferry S, Hotopf M, Hull L, Ismail, K, Palmer I, David A, Wessely S. Health of UK servicemen who served in Persian Gulf War. Lancet, 353:169-178, January 16, 1999.
Wolfe J, Proctor SP, Davis JD, Borgos MS, Friedman MJ. Health symptoms reported by Persian Gulf War veterans two years after return. Am J of Industrial Med, 33:104-113, 1998.
back to top
VIII. BIBLIOGRAPHY
Alloway JA, Older SA, Battafarana DF, Carpenter MT. Persian Gulf War myalgia syndrome. J Rheumatol, 25(2):388-9, 1998.
Amato AA, Jackson C, McVey A. Identification of Gulf War syndrome: methodological issues and medical illnesses. JAMA, 278(5):384-5; discussion 385-7, 1997.
Amato AA, McVey A, Cha C, Matthews EC, Jackson CE, Kleingunther R, Worley L, Cornman E, Kagan-Hallet A. Evaluation of neuromuscular symptoms in veterans of the Persian Gulf War. Neurology, 48(1):4-12, January 1997.
Andres C, Beeri G, Friedman A, Lev-Lehman E, Henis S, Timberg R, Shani M, Soreq H. Acetylcholinesterase-transgenic mice display embryonic modulations in spinal chord choline acetyltransferase and neurexin 1b gene expression followed by late-onset neuromotor deterioration. Proc Natl Acad Sci, USA, 94(15)8173-8, 1997.
Andres C, Seidman S, Beeri R, Timberg R, Soreq H. Transgenic acetylcholinesterase induces enlargement of murine neuromuscular junctions but leaves spinal cord synapses unchanged. Neurochem Int, 32:449-56, 1998.
Anger WK, Rohlman DS, Storzbach D. Neurobehavioral Testing in Humans. Current Protocols in Toxicology (M Maines, L Costa, IG Sipes, S Sasse, DJ Reed, eds). John Wiley & Sons, New York, (in press).
Anger WK, Storzbach D, Amler RW, Sizemore OJ. Human behavioral neurotoxicology: Workplace and community assessments. In: Rom W, Environmental and Occupational Medicine, 3rd edition, Philadelphia, Lippincott-Raven pp. 709-31.
Anger WK, Storzbach D, Binder LM, Campbell KA, Rohlman DS, McCauley L, Kovera CA, Davis KL. Neurobehavioral Deficits in Persian Gulf Veterans: Evidence from a population-based study. Journal of the International Neuropsychology Society, (in press).
Araneta MRG, Moore CA, Olney RS, Edmonds LD, Karcher JA, McDonough C, Hiliopoulos KM, Schlangen KM, Gray GC. Goldenhar syndrome among infants born in military hospitals to Gulf War veterans. Teratology, 56;244-51, 1997.
Army Research Laboratory Technical Report ARL-TR-800, July 1995.
Axelrod BA, Milner IB. Neuropsychological findings in a sample of Operation Desert Storm veterans. J Neuropsychiatry Clin Neurosci, 9:23-8, 1997.
Baker DG, Boat B, Grinvalsky HT, Geracioti TD. Interpersonal and Animal Related Trauma in Female and Male Military Veterans, Mil Med, 163:20-5, 1998.
Baker DG, Mendenhall CL, Simbartl LA, Megan LK, Steinberg JL. Relationship between Posttraumatic Stress Disorder and Self-Reported Physical Symptoms in Persian Gulf War Veterans, Archives of Medicine, 157:2076-8, 1997.
Baker DG, West SA, Orth DN, Hill KK, Nicholson WE, Ekhator NN, Bruce AB, Wortman MD, Keck PE, Geracioti TD. Cerebrospinal Fluid and Plasma-Endorphin in Combat Veterans with Posttraumatic Stress Disorder. Psychoneuroendocrinology, 22:517-29, 1997.
Barkhuizen A, Rosen H, Wolf S, Flora K, Benner K, Bennett R, Musculoskeletal pain and fatigue is associated with chronic hepatitis C: a report of 239 hepatology clinic patients. Am J Gastroenterol, (in press).
Baynes RA, Monteiro-Riviere NA, Qiao GL, Riviere JE: Cutaneous toxicity of the benzidine dye Direct Red 28 applied as a mechanistically-defined chemical mixture (MDCM) in perfused porcine skin. Toxicology Letters, 93:159-69, 1997.
Baynes RE, Halling KB, Riviere JE. The influence of diethyl-m-toluamide (DEET) on the percutaneous absorption of permethrin and carbaryl. Toxicol Appl Pharmacol, 144(2):332-9, 1997.
Beeri R, Le Novere Mervis R, Huberman T, Grauer E, Changeux JP, Soreq H. Enhanced hemicholinium binding and attenuated dendrite branching in cognitively impaired acetylcholinesterase-transgenic mice. J Neurochem, 69:2441-51, 1997.
Bell IR, Patarca R, Caldwin CM, Klimas NG, Schwartz GE, Hardin EE. Serum neopterin and somatization in women with chemical intolerance, depressives, and normals. Neuropsychobiology, 38(12):13-8, 1998.
Bell IR, Warg-Damiani L, Baldwin CM, Walsh ME, and Schwartz GE. Self-reported chemical sensitivity and wartime chemical exposures in Gulf War veterans with and without decreased global health ratings. Mil Med, 163(11):725-32, 1998.
Bennet RM, Clark SC, Walczyk J. A randomized, double-blind, placebo-controlled study of growth hormone in the treatment of fibromyalgia. Am J Med, 104:227-34, 1998.
Benotsch E, Brailey K, Vasterling JJ, Uddo M, Constans JI, Sutker PB. Longitudinal relationship of individual resource factors to stress-related psychopathology, (in press).
Benschop HP, Van der Schans GP, Noort D, Fidder A, Mars-Groeneudijk RH, De Jong LPA. Verification of exposure to sulfur mustard in two casualties of the Iran-Iraq conflict. J Anal Toxicol, 21:249-51, 1997.
Bernstein JA, Martin RLM, Lummus ZL. Localized Human Seminal Plasma Hypersensitivity: A Potential Model for Gulf War 'Burning Semen Syndrome'. Fundam Appl Toxicol, 36:201, 1997.
Berry C. The Gulf War syndrome. J Clin Pathol, 50(5):360, 1997.
Binder LM, Storzbach D, Anger WK, Campbell KA, Rohlman DS, & members of the Portland Environmental Hazards Research Center. Subjective cognitive complaints, affective distress, and objective cognitive performance in Persian Gulf War veterans. Clin Neuropsychol, (in press).
Brailey K, Vasterling JJ, Sutker PB. Psychological aftermath of participation in the Persian Gulf War. In A. Lundberg (ed). The Environment and mental health: a guide for clinicians. Lawrence Earlbaum: Mahwah, NJ, 83-101, 1998.
Buccafusco JJ, Prendergast MA, Pauly JR, Terry AV, Goldstein BD, Shuster LC. A rat model for Gulf War Illness-related selective memory impairment and the loss of hippocampal nicotinic receptors. Soc Neurosci Abs, 23:216, 1997.
Callahan HL, Grogl M. Development of animal models to study Leishmania tropism at the molecular level. Am J Trop Med Hyg, (in press).
Callahan HL, Portal IF, Bensinger SJ, Grogl M. The temperature sensitivity of Leishmania promastigotes in vitro: a model for tropism in vivo. Exp Parasitol, 84:400-9, 1996.
Campbell KA, Rohlman DS, Storzbach D, Binder LM, Anger, WK, Kovera CA, Davis K, Grossmann S. Test-retest reliability of psychological and neurobehavioral tests self-administered by computer. Assessment, 6(1)21-32, 1999.
Cannova JV. Multiple giant cell tumors in a patient with Gulf War syndrome. Mil Med, 163(3):184-5, 1998.
Chaney LA, Rjockhold RW, Mozingo JR, Hume AS, and Moss JI. Potentiation of pyridostigmine bromide toxicity in mice by selected adrenergic agents and caffeine. Vet Hum Toxicol, 39(4):214-9, 1997.
Clapp, R. Update of cancer surveillance of veterans in Massachusetts. Letter to the Editor; Int J Epidemiol, 26(3):679-81, 1997.
Clauw D, Chrousos GP. Chronic Pain and Fatigue Syndromes: Overlapping Clinical and Neuroendocrine Features and Potential Pathogenic Mechanisms. NeuroImmunomodulation, 4;134-53, 1997.
Clauw D. The 'Gulf War Syndrome'; Implications for Rheumatologists. J Clin Rheumatol, 4(4);173-4, 1998.
Coker WJ, Bhatt BM, Blatchley NF, Graham JT. Clinical findings for the first 1000 Gulf war veterans in the Ministry of Defence’s medical assessment programme. BMJ, 318:290-4, 1999.
Combat service support survey results: A light infantry division and a mechanized infantry division. Technical Report AD#ADA278203.
Cowan DN, DeFraites RF, Gray GC, Goldenbaum M, Wishik S. The risk of birth defects among children of Persian Gulf War veterans. N Engl J Med, 336:1650-6, 1997.
Cowan DN, DeFraites RF, Gray GC, Goldenbaum M. Birth defects among children of Gulf War veterans. Amer J Epidemiol, 145:512, 1997, Abstract.
Cowan DN, Gray GC, DeFraites RF. Counterpoint: Responding to inadequate critique of birth defects paper. Amer J Epidemiol, 148:326-7, 1998.
Cowan DN, Gray GC, DeFraites RF. The authors reply to 'Birth defects among children of Persian Gulf War veterans.' N Engl J Med, 337:1175-6, 1997, Letter.
Das AK, Davanzo LD, Poiani GJ, Zazzali PG, Scardella AT, Warnock ML, Edelman NH. Variable extra thoracic airflow obstruction and chronic laryngotracheitis in Gulf War veterans. Chest, 115(1):97-1, 1999.
David A, Ferry S, Wessely S. Gulf war illness. BMJ, 314(7076):239-40, 1997.
Dillon DC, Day CH, Whittle JA, Magill AJ, Reed SG. The molecular and immunological characterization of Leishmania tropica antigens: serological and cellular responses by viscerotropic Leishmaniasis Patients. VIII Intern Congress of Parasitology, 1994.
Dillon DC, Day CH, Whittle JA, Magill AJ, Reed. Characterization of a Leishmania tropica antigen that detects immune responses in Desert Storm Viscerotropic Leishmaniasis patients. Proc Natl Acad Sci, (92)7981-2, 1995.
Draft Environmental Surveillance Health Risk Assessment, Kuwait Oil Fires: http://www.gulflink.osd.mil/envexp.html
Drake-Baumann R, Seil FJ, Spencer PS. Neuromuscular responses to pyridostigmine bromide in organotypic spinal cord-muscle culture. J Physiol, (Paris), 92:305-8, 1998.
Drake-Baumann R, Seil FJ. Effects of long-term exposure to pyridostigmine on neuromuscular junctions in vitro, (submitted).
Engel CC Jr, Jing Z. Identification of Gulf War syndrome: methodological issues and medical illnesses. JAMA, 273(5):383-4; discussion 385-7, 1997.
Engel CC Jr, Roy M, Kayanan D, Ursano R. Multidisciplinary treatment of persistent symptoms after Gulf War service. Mil Med, 163(4):202-8, 1998.
Environmental Exposure Report, Depleted Uranium in the Gulf: http://www.gulflink.osd.mil/du
Environmental Exposure Report, Oil Well Fires: http//www.gulflink.osd.mil/envexp.html
Epidemiological considerations regarding the health and effectiveness of women in the armed forces. Technical Report, AD#ADA278202.
Fidder A, Noort D, Benshop HP. A convenient synthesis of [14C],1-thiobis(2-chloroethane), [1+ C]sulfur mustard. J Label Comp Radiopharm, (in press).
Fiedler N, Kipen H, Natelson B, Ottenweller J. Chemical sensitivities and the Gulf War: Department of Veterans Affairs Research Center in Basic and Clinical Studies of Environmental Hazards. Regul Toxicol Pharmacol, 24:129-38, 1996.
Fiedler N, Kipen H. Multiple Chemical Sensitivity, Chronic Fatigue Syndrome, and the Gulf War. Toxicol Ind Health 1998, (in press).
Frank AF, Blythe LL, Spencer PS. Aspects of veterinary neurotoxicology. In: Spencer PS, Schaumburg HH, eds, Experimental and Clinical Neurotoxicology, Oxford University Press, New York; 1999, (in press).
Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, Mawle AC, Reeves WC. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. JAMA, 280(11):981-8, 1998.
Gattu M, Boss K, Terry A, Buccafusco J. Reversal of scopolamine-induced deficits in navigational memory performance by the seed oil of celastrus paniculatus, (57):4:793-9, 1997.
Geracioti TD, Goldsmith JR, Friedman LM, Norman AB, Somoza E, Kasckow JW, Baker DG, Richtand NM, Anthenelli RM, Keck PE. Cerebrospinal fluid neuroendocrinology of alcohol misusers. Addic Biol, 2:401-9, 1997.
Geracioti, TD, Loosen PT, Ekhator NN, Schmidt D, Chambliss B, Baker DG, Kaskow JW, Richtand NM, Keck PE, Ebert MH. Uncoupling of Serotonergic and Noradrenergic Systems in Depression: Preliminary Evidence from Continuous Cerebrospinal Fluid Sampling. Depress Anxiety, 6(3)89-94, 1997.
Gold BG, Schnell J, Spencer PS. Neurotoxic Disorders. In: Atlas of Clinical Neurology. (ed. Rosenberg RN), Butterworth-Heinemann, Philadelphia, (14)22;1-14, 1998.
Goldstein G, Beers SR, Morrow LA, Shemansky WJ, Steinhauer SR. A preliminary neuropsychological study of Persian Gulf veterans. J Int Neuropsychol Soc, 2(4)368-71, 1996.
Gordon JM, Verne GN, Davis RH, Sninsky CA, Gainesville VAMC, Dept of Med Univ of FL, Gainesville, and Dept of Med, MUSC, Charleston SC. Is the diarrhea in Persian Gulf war veterans caused by an underlying visceral hypersentivity? Biomedicine, 1996.
Grady EP, Carpenter MT, Koenig CD, Older SA, Battafarano DF. Rheumatic findings in Gulf War veterans. Arch Intern Med, 158(4):367-71, 1998.
Gray GC, Coate BD, Anderson CM, Kang HK, Berg SW, Wignall FS, Knoke JD, Barrett-Connor E. The postwar hospitalization experience of US Persian Gulf War veterans. N Engl J Med, 335:1505-13, 1996.
Gray GC, Hawksworth AW, Smith TC, Kang HK, Knoke JD, Gackstetter GD. Gulf War veterans' health registries. Who is most likely to seek evaluation? Am J Epidemiol, 148:343-9, 1998.
Gray GC, Hawksworth AW, Tyler CS, Kang, HK, Knoke JD, and Gackstetter GD. Gulf War Veterans’ Health Registries. Who is most likely to seek evaluation? Am J Epidemiol, 148(4):343-9, 1998.
Gray GC, Kaiser KS, Hawksworth AW, Hall FW, Barrett-Connor EL. Increased postwar symptoms and psychological morbidity among US Navy Gulf War veterans. Under external review.
Gray GC, Kaiser KS, Hawksworth AW, Watson HL. No serological evidence of an association found between Gulf War service and Mycoplasma fermentans infection. Am J Trop Med Hyg, 1998, (in press).
Gray GC, Knoke JD, Berg SW, Wignall FS, Barrett-Connor E. Counterpoint: Responding to suppositions and misunderstandings. Am J Epidemiol, 148:328-33, 1998.
Greene DA. Locked and loaded. Roger Castle’s Gulf War syndrome. N C Med J, 58(1):48-51, 1997.
Grifman M, Gralyam N, Seidman S, Soreq H. Functional redundancy of acetylcholinesterase and neuroligin in mamalian neuritogenesis. Proc Natl Acad Sci, 95:13935-40, 1998.
Grisaru D, Lev-Lehman E, Shapira M, Chikin E, Lessing LB, Eldor A, Eckstein F, Soreq H. Human osteogenesis involves differentiation -dependent increases in the morphogenically active 3 alternative splicing variant of acetylcholinesterase. Molec Cell Biol, 19(1)788-95, 1999.
Haley RW, Kurt TL. Self-reported exposure to neurotoxic chemical combinations in the Gulf War: A cross-sectional epidemiologic study. JAMA, 277:231-7, 1997.
Haley RW, Kurt TM, Hom J. Is there a Gulf War syndrome? Searching for syndromes by factor analysis of symptoms. JAMA, 277:215-22, 1997.
Haley RW, Kurt TM, Roland PS, et al. Evaluation of neurologic function in Gulf War veterans: a blinded case-control study. JAMA, 277:223-30, 1997.
Haley RW. Is Gulf War syndrome due to stress? The evidence reexamined. Am J Epidemiol, 146:695-703, 1997. (See correspondence and author's replies Am J Epidemiol, 148:402-70, 1998).
Haley RW. Point: Bias from the "healthy warrior effect" and unequal followup in government studies of the health effects of the Gulf War. Amer J Epidemiol, 148(4):315-23, 1998.
Heaf P, Jones N. Gulf War illness. Family doctors should be aware of assessment programme. BMJ, 314(7086):1041, 1997.
Hebert M, Potegal M, Moore T, Evenson AR, Meyerhoff J. Diazepam exacerbates conditioned defeat in hamsters (Mesocricetus auratus). Pharmacol Biochem Behav, 55:405-13, 1996.
Heinzel FP, Jujer AM, Ahmed F, Rerko RM. The in vivo production and function of IL-12 p40 homodimers. J Immunol, 158:4381-8, 1997.
Heinzel FP, Rerko RM, Jujer AM, Maier RA. Increased interleukin-2 synthetic capacity parallels disease progression in mice infected with Leishmania major. Infect Immun, 66:4537-40, 1998.
Heinzel FP, Rerko RM, Jujer AM. Underproduction of interleukin-12 during progressive murine leishmaniasis is due to decreased CD40 activity. Cell Immunol, 184:129-42, 1998.
Heinzel FP. From oriental sore to Kala-azar: Clinical and therapeutic aspects of the Leishmaniasis. Clin Microbiol, Newsletter, 19:121-6, 1997.
Hodgson M, Kipen H, Puglisi M. Gulf War illness: causation and treatment. J Occup Environ Med 1998; Accepted, pending revisions.
Hogan J, Benson KA, Landauer M, Pellmar TC. Toxicity of embedded Depleted Uranium (DU) in the rat. Toxicologist, 36:17, 1997.
Horn J, Haley RW, Kurt TL. Neuropsychological correlates of Gulf War syndrome. Arch Clin Neuropsychol, 12:531-44, 1997.
Hoy JB, Cody BA, Karlix JL, Schmidt CJ, Tebbett IR, Toffollo S, vanHaaren F, Wielbo D. Pyridostigmine bromide alters locomotion and thigmotaxis of rats: gender effects. Pharmacol Biochem Behav, 1998 (in review).
Hyams KC, Roswell RH. Resolving the Gulf War syndrome question. Am J Epidemiol, 148(4):339-42, 1998.
Hyams KC. Lessons derived from evaluating Gulf War syndrome: suggested guidelines for investigating possible outbreaks of new diseases. Psychosom Med, 60(2):137-9, 1998.
Hyman ES, Deming QB. Urinary sediment examination and Gulf War Syndrome. Am J Med Sci, 316(6):411-3, 1998.
Hyman ES. A computer algorithm offers a comprehensive view of quantitative bacteriuria. Nephron, 65:549-58, 1993.
Hyman ES. A Urinary Marker for Occult Systemic Coccal Disease. Nephron, 68:314-26, 1994.
Hyman ES. Improved microscopic detection of bacteriuria. Biotech Histochem, ( 27)1:1-8, 1992.
Ignatushchenko M, Winter R, Hinrichs D, Riscoe M. Antileishmanial drug design - Chemotherapeutic exploitation of parasite heme dependency, (under revision).
Ignatushchenko M, Winter RW, Hinrichs DJ, Riscoe MK. Xanthones as antimalarial agents: Studies of a possible mode of action. FEBS Letters, 409:67-73, July 1997.
Ignatushchenko M, Winter RW, Riscoe M. Xanthones as Antimalarial Agents; Stage-specific action and structure-activity relationships, (submitted).
Iowa Persian Gulf Study Group. Self-reported illness and health status among Gulf War veterans: a population-based study. JAMA, 277:238-45, 1997.
Ismail K, Everitt B, Blatchley N, Hull L, Unwin C, David A, Wessely S. Is there a Gulf War syndrome? Lancet, 353:179-82, 1999.
Jamal GA, Aziz MA. Gulf War syndrome—is it a disease of complex interaction of several factors? CME Bulletin Medical Microbiology, 2(3):62-4, 1998.
Jamal GA. Gulf War syndrome—a model for the complexity of biological and enviornmental interaction with human health. Adverse Drug React Toxicol Rev, 17(1)-17. Review, March 1998.
Joellenbeck LM, Landrigan RJ, Larson EL. Gulf War veterans’ illnesses: a case study in causal inference. Environ Res, 79(2):71-81, 1998.
Joseph SC, Hyams KC, Gackstetter, GD, Mathews EC, and Patterson RE. Persian Gulf War health issues. Environ Occ Med, 3rd Ed, 1595-610, 1988.
Kaiser KS, Hawksworth AW, Gray GC. Pyridostigmine bromide intake during the Persian Gulf War not associated with postwar handgrip strength. Under external review.
Kang H, Bullman TA. Mortality among U.S. veterans of the Persian Gulf war. N Engl J med, 1996, 335:1498-504.
Karczmar A. Invited review: Anticholinesterases: dramatic aspects of their use and misuse. Neurochem Int, 32(5-6):401-11. Review, 1998.
Kaufer D, Friedman A, Scidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature, 393:373-7, 1998.
Kaufer D, Friedman A, Seidman S, Soreq H. Anticholinesterase induce multigenic transcriptional feedback response suppressing cholinergic neurotransmission. Chem Biol Interact, (in press).
Kaufer D, Friedman A, Soreq H. The vicious circle: long lasting transcriptional modulation of cholinergic neurotransmission following stress and anticholinesterase exposure. Neuroscientist, (in press).
Kellner M, Baker DG, Yehuda R. Salivary Cortisol in Operation Desert Storm Returnees. Biological Psychiatry, 42:849-50, 1997.
Kelly DJ, Chan CT, Paxton H, Thompson K, Howard R, Dasch GA. Comparative evaluation of a commercial enzyme immunoassay for the detection of human antibody to Rickettsia typhi. Clin Diagn Lab Immunol, 2(3):356-60, 1995.
Kelly JX, Winter RW, Riscoe MK, Peyton DH. Xanthones as antimalarial agents: Binding interactions between heme analogues and xanthones, (submitted).
Khan ZU, Neil L, Chandy R, Chugh TD, Al-Sayer H, Provost F, Boiron P. Nocardia asteroides in the soil of Kuwait. Mycopathologia, 137(3):159-63, 1997.
Kipen H, Hallman, Kang, Fiedler, Natelson. Prevalence of Chronic Fatigue and Chemical Sensitivities in Gulf Registry Veterans. Archives of Environmental Health, (submitted).
Kisby GE, Springer N, Spencer PS. In Vitro neurotoxic and DNA-damaging properties of nitrogen mustard (HN2), (in press).
Kisby GE, Sweatt, Spencer PS. Role of DNA repair in protecting mature nervous tissue from DNA damage. J Cell Biochem 21A, 348, 1995.
Klaustermeyer WB, Kraske GK, Lede KG, Kurohara ML. Allergic and immunologic profile of symptomatic Persian Gulf War veterans. Ann Allergy Asthma Immunol, 80(3):269-73, 1998.
Knoke JD, Gray GC, Garland FC. Testicular cancer and Persian Gulf War service. Epidemiology, 9(6):648-53, 1998.
Knoke JD, Gray GC, Garland FC. Lack of association of testicular cancer with Persian Gulf War service. Epidemiology, 9:648-53, 1998.
Knoke JD, Gray GC. Hospitalizations after the Gulf War - Reply to K.M. Leisure, et al. Emerging Infectious Diseases. 4:709, 1998, Letter.
Knoke JD, Smith TC, Gray GC, Kaiser KS, Hawksworth AW. Factor analysis: Does it identify a Gulf War syndrome? Under external review.
Knoke JD. And Gray GC. Hospitalizations for unexplained illnesses among U.S. veterans of the Persian Gulf War. Emerg Infect Dis, 4(2):211-9, 1998.
Korenyi-Both AL, Korenyi-Both AL, Juncer DJ. Al Eskan disease: Persian Gulf syndrome. Mil Med, 162(1):1-13, 1997.
Kounios J, Litz BT, Kaloupek DG, Riggs D, Knight J, Weathers FW, Anderson J, Keane TM. Electrophysiology of combat-related PTSD. Ann N Y Acad Sci, 821:504-7, 1997.
Krengel M, White RF, Diamond R, Letz R, Cyrus P, Durso R. A comparison of NES2 and traditional neuropsychological tests in a neurologic patient sample. Neurotoxicol Teratol, 18:435-9, 1996.
Kroenke K, Kowlowe P, and Roy M. Symptoms in 18495 Persian Gulf War veterans. Latency of onset and lack of association with self-reported exposures. J Occup Environ Med, 40(6):520-8, June 1998.
Landrigan PJ, Lashof JC, Hamburg DA. Re: Is Gulf War syndrome due to stress? The evidence reexamined. Am J Epidemiol, 148(4):404-7, 1998.
Landrigan PJ. Illness in Gulf War veterans. Causes and consequences. JAMA, 277(3):259-61, 1997.
Lashof JC, Cassells JS. Illness among Gulf War veterans: risk factors, realities, and future research. JAMA, 280(11):1010-1, 1998.
Li L, Gunasekar PG, Borowitz JL, Isom GE. Pyridostigmine-induced neuronal apoptosis, (submitted for publication).
Liu W, Hillman AG, Harmon JM. Hormone-independent repression of AP-1 inducible collagenase promoter activity by glucocorticoid receptors. Mol Cell Biol, 15:1005-13, 1995.
Ludolph AC, Spencer PS. Toxic neuropathies and their treatment. In: Peripheral Neuropathies, Part 2, Balliere's Clinical Neurology; International Practice and Research (ed. Hartung HP), Balliere, London, 1996.
Marino MT, Schuster BG, Brueckner RP, Lin E, Kamainskis A, Lasseter R. Population pharmacokinetics and pharmacodynamics of pyridostigmine bromide for prophylaxis against nerve agents in humans. J Clin Pharmacol, 38(3):227-35, 1998.
McCauley LA, Joos SK, Spencer PS, Lasarev MR, Shuell T, Other Members of the Portland Environmental Hazards Research Center. Strategies to assess validity of self-reported exposures during the Persian Gulf War, (submitted).
Medinger AE, Chan TW, Arabian A, Rohatgi PK. Interpretive algorithms for the symptom-limited exercise test: assessing dyspnea in Persian Gulf war veterans. Chest, 113(3):612-8, March 1998.
Melby PC, Tryon VV, Chandrasekar B, Freeman GL. Cloning of Syrian hamster (Mesocricetus auratus) cytokine cDNAs and analysis of cytokine mRNA expression in experimental visceral leishmaniasis. 1998, (manuscript submitted).
Melby PC, Yang YZ, Cheng J, Zhao W. Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun, 66:18-27, 1998.
Miller AC, Blakely WF, Livengood D, Whittaker T, Xu J, Ejnik JW, Hamilton MM, Parlette E, St. John T, Gersterbers HM, and Hsu H. Transformation of human osteoblast cells to the tumorigenic phenotype by depleted uranium-uranyl chloride, 106(8):465-71, 1998.
Miller AC, Fuciarelli AF, Ejnik EJ, Strocko S, Hogan J, Page N, Pellmar T. Urinary and serum mutagenicity studies with rats implanted with depleted uranium or tantalum pellets. Mutageneis, 13:643-8, 1998.
Morbidity and Mortality Weekly Report: Unexplained Illness Among Persian Gulf War Veterans in an Air National Guard Unit, Preliminary Report, August 1990-March 1995. MMWR, 44(23):443-7, 1995.
Nicolson GL, Nicolson NL. Gulf War illnesses: complex medical, scientific and political paradox. Med Confl Surviv, 14(2):156-65, 1998.
Nicolson GL, Nicolson NL. The eight myths of operation ‘Desert Storm’ and Gulf War syndrome. Med Confl Surviv, 13(2):140-6, 1997.
Noort D, Hulst AG, Trap HC, De Jong LPA, Benschop HP. Synthesis and mass spectrometric identification of the major amino acid adducts formed between sulphur mustard and hemoglobin in human blood. Arch Toxocol, 71:171-8, 1997.
Orr SP, Kaloupek DG. Psychophysiological assessment of PTSD. In J.P. Wilson & T.M. Keane (Eds.) Assessing Psychological Trauma and PTSD (pp. 69-97). New York: Guilford, 1996.
Oversteet DH, Daws LC, Schiller GC, Orbach J, Janowsky DS. Cholinergic/serotonergic interactions in hypothermia: Implications for rat models of depression. Pharmacol Biochem Behav, 59:777-85, 1998.
Peacock MD, et al. Sleep apnea-hypopnea syndrome in a sample of veterans of the Persian Gulf War. Mil Med, 162(4):249-51, 1997.
Pellmar TC, Fuciarelli AF, Ejnik JW, Hamilton M, Hogan J, Strocko S, Emond C, Mottaz HM, Landauer MR. Distribution of uranium in rats implanted with depleted uranium pellets. Toxicol
Sciences, (in press).
Pellmar TC, Hogan J, Benson KA, Landauer M. Health Risk Assessment of Embedded Depleted Uranium (DU) 6-Month Evaluation Point, AFRRI Special Publication Report, 97-4, 1997.
Pellmar TC, Hogan JB, Benson KA, Landauer MR. Depleted uranium distribution and toxicology in a rodent model. AFRRI Special Publication Report, 98-3:7-9, 1998.
Penman AD, Tarver RS. No evidence of increase in birth defects and health problems among children born to Persian Gulf War veterans in Mississippi. Mil Med, 161(1)1-5, 1996.
Pitman RK, Orr SP, van der Kolk BA, et al. Analgesia: a new dependent variable for the biological study of PTSD, In Post Traumatic Stress Disorder: Etiology Phenomenology and Treatment (ed by Wolf ME). American Psychiatric Press, 141-7, 1990.
Poirier MC, Weston A, et al. Biomonitoring of United States Army Soldiers Serving in Kuwait in 1991. Cancer Epidemiol Biomarkers & Prev, 7(6)545-51, 1998.
Pollet C, Natelson B, Lange G, Tiersky L, DeLuca J, Policastro T, et al. Medical evaluation of Persian Gulf veterans with fatigue and/or chemical sensitivity. J Med, 29(3-4):101-13, 1998.
Potegal M, Ferris C, Hebert M, Skaredoff L, Meyerhoff J. Attack priming in female Syriahn golden hamsters is mediated by a c-fos coupled process within the corticomedial amygdala. Neuroscience, 75:869-80, 1996.
Potegal M, Hebert M, deCoster M, Meyerhoff J. Brief, high frequency stimulation of the corticomedial amygdala induces a delayed and prolonged increase of aggressiveness in male Syrian golden hamsters. Behav Neurosci, 110(2):401-12, 1996.
Prendergast M, Terry A, Buccafusco J. Chronic, low-level exposure to diisopropylfluorophosphate causes protracted impairment of spatial navigation learning. Psychopharmacology, 129:183-91, 1997.
Prendergast MA, Buccafusco JJ. (-)Nicotine exposure increases mRNA encoding G3PDH and the vesicular acetycholine transporter in vivo. Neuro Reports 9:1385-9, 1998.
Prendergast MA, Terry AV, Buccafusco JJ. Effects of chronic, low-level organophosphate exposure on delayed recall, discrimination, and spatial learning monkeys and rats. Neurotoxicol
Teratol, 20:115-22, 1998.
Proctor SP, Heeren T, White RF, Wolfe J, Borgos MS, Davis JD, Pepper L, Clapp R, Sutker PB, Vasterling JJ, Ozonoff D. Health status of Persian Gulf War veterans: self-reported symptoms, environmental exposures and the effect of stress. Int J Epidemiol, 27(6)1000-10, 1998.
Ramdas J, Harmon JM. Glucocorticoid-induced apoptosis and regulation of NF-B activity in human leukemic T cells. Endocrinology, 139:3813-21, 1998.
Ramdas J, Liu W, Harmon JM. glucocorticoid-induced cell death requires autoinduction of glucocorticoid receptor expression in human leukemic T cells. Cancer Res, 59(6)1378-85, 1999.
Returning Persian Gulf Troops: First Year Findings, published by Northeast Program Evaluation Center (NEPEC), VAMC, West Haven, Connecticut, 1992.
Rook GA, Zumla A. Is the Gulf War syndrome an immunologically mediated phenomenon? Hosp Med, 59(1):10-1, 1998.
Rook GA, Zumla A. Gulf War syndrome: is it due to a systemic shift in cytokine balance towards a Th2 profile? Lancet, 349(9068)1831-3, 1997.
Roy MJ, Koslowe, PA, Kroenke K, Magruder C. Signs, symptoms, and ill-defined condition in Persian Gulf War Veterans: findings from the comprehensive clinical evaluation program. Psychosom Med 60:663-8. Editorial Comment 669-670, 1998.
Sang DK, Ouma JH, Whalen CC, King C, Mahmoud AAF, Heinzel FP. Increased levels of soluble interleukin-4 receptor in serum of patients with visceral leishmaniasis. J Infect Dis, 179(3)743-6, 1999.
Sapolsky RM. The stress of Gulf War syndrome. Nature, 393(6683):308-9, 1998.
Schlesinger N, Baker DG, Schumacher HR Jr. Persian Gulf War myalgia syndrome. J Rheumatol, 24(5):1018-9, 1997.
Schwartz DA, Doebbeling BN, Merchant JA, et al. Self-reported illness and health status among Gulf War veterans: A population-based study. JAMA, 1997, 277:238-45.
Servatius R, Ottenweller J, Beldowicz D, Guo W-D, Zhu G. Persistently exaggerated startle responses in rats treated with pyridostigmine bromide. J Pharmacol Exp Ther, 287:1020-8, 1998.
Shen ZX. Pyridostigmine bromide and Gulf War syndrome. Med Hypotheses, 51(3):235-7, 1998.
Sivo J, Harmon JM, Vogel SN. Heat shock mimics glucocorticoid effects on IFN-induced FcRI and Ia mRNA expression in mouse peritoneal macrophages. J Immunol, 156:3450-4, 1996.
Sloan P, Aresenault L, Hilsenroth M. A longitudinal evaluation of the Mississippi Scale for Combat-related PTSD in detecting war-related stress symptomatology. J Clin Psychol, (54)8:1085-90, 1998.
Sloan P, Arsenault L, Hilsenroth M, Harvill L, Handler L. Rorschach measures of post-traumatic stress in Persian Gulf War veterans. J Pers Assess, 64, 397-414. 1995.
Sloan P, Arsenault L, Hilsenroth M, Harvill L. Assessment of non-combat, war-related post-traumatic stress symptomatology: validity of the PK, PS, and IES Scales. Assessment, (3)1, 37-41, 1996.
Sostek M. High prevalence of chronic gastrointestinal symptoms in a national guard unit of Persian Gulf veterans. Am J of Gastroent, 91(12)2494-7, 1996.
Southern PM Jr, Patel S, Gander RM. Does examination of urinary sediment identify individuals with Gulf War syndrome? A pilot study. Am J Med Sci, 315(4):225-9, 1998.
Spencer PS, Daniels J, Kisby GE. Mustard warfare agents and related substances. In: Spencer PS, Schaumburg HH, eds, Exper Clin Neurotoxicol. New York: Oxford University Press; 1999, (in press).
Spencer PS, Schaumburg HH, Ludolph AC. Experimental and Clinical Neurotoxicology, 2nd edition, Oxford, New York, (in press).
Spencer PS, Wilson B, Albuquerque EX. Sarin, other "nerve agents" and their antidotes. In: Spencer PS, Schaumburg HH, eds, Experimental and Clinical Neurotoxicology, New York: Oxford University Press; 1999, (in press).
Steinhauer SR, Beers SR, Goldstein G, Shemansky WJ, Morrow LA. Neurophysiological and neuropsychological characteristics of Gulf War veterans. J Intern Neurop Soc, (abstract) 2:68, 1996.
Sternfeld M, Ming G, Song H, Sela K, Timberg R, Poo M, Soreq H. Acetylcholinesterase enhances neurito growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J Neurosci, 18(4):1240-9, 1998.
Sternfeld M, Patrick JD, Soreq H. Position effect variegations and brain-specific silencing in transgenic mice overexpressing human acetylcholinesterase variants. J Physiol (Paris), 92:249-56, 1998.
Stretch RH, Bliese PD, Marlowe DH, et al. Physical health symptomatology of Gulf War-era service personnel from the states of Pennsylvania and Hawaii. Mil Med, 160:131-6, 1995.
Stretch RH, Marlowe DH, Wright KM, et al. Post-traumatic stress disorder symptoms among Gulf War veterans. Mil Med, 161:407-10, 1996.
Taylor DN, Sanchez JL, Smoak BL, DeFraites R. Helicobacter pylori infection in Desert Storm troops. Clin Infect Dis, 25(5):979-82, 1997.
Stuart JA, Bliese PD. The Long Term Effects of Operation Desert Storm on the Psychological Well-Being of US Army Reserve and National Guard Veterans. J Appl Soc Psychol, 28(1)1-22, 1998.
Tiersky L, Natelson B, Ottenweller J, Lange G, Fiedler N, DeLuca J. Functional status and mood in Persian Gulf veterans with unexplained fatiguing illness. Mil Psychol, 1998; Accepted, pending minor revisions.
Unwin C, Blatchley N, Coker W, Ferry S, Hotopf M, Hull L, Ismail, K, Palmer I, David A, Wessely S. Health of UK servicemen who served in Persian Gulf War. Lancet, 353:169-78, 1999.
Unwin CE, Blatchley N, Coker W, Ferry S, Hotopf M, Hull L, Ismail K, Palmer I, David A, Wessely S. The health of United Kingdom Servicemen who served in the Persian Gulf War. Lancet, 353:169-78, 1999.
US Army Center for Health Promotion and Preventive Medicine Report. Acute Oral Toxicity Study of Pyridostigmine Bromide, Permethrin, and DEET in the Laboratory Rat. Toxicological Study, 75-48-2665, 1995.
US Army Environmental Hygiene Agency, Final Report. Kuwait Oil Fire Health Risk Assessment. No. 39-26-L192-91, 1994.
Vandenborne K, Walter G, Lenrow D, Leigh JS, Fishman AP. Skeletal muscle in Persian Gulf veterans. J Chronic Fatigue Syndrome, 2(2/3):137-8, 1996.
vanHaaren F, de Jongh R, Hoy JB, Karlix JL, Schmidt CR, Tebbett Ir, Wielbo D. The effects of acute and repeated pyridostigmine bromide adminstration on response aquisition with immediate and delayed reinforcement. Pharmacol Biochem Behav, 62(2)389-94, 1999.
Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychol, 12:125-33, 1998.
Vaziri C, Shneider A, Sherr DH, Faller D. Expression of the aryl hydrocarbon receptor is regulated by serum and mitogenic growth factors in murine 3T3 fibroblasts. J Biol Chem, 271, 25921-7, 1996.
Veggeberg S. Unexplained illnesses not unique to Gulf War veterans. Mol Med Today, 1(7):199, 1995.
Wallace H, Natelson B, Gause W, Hay J. Human herpesviruses in chronic fatigue syndrome. Clin Diagn Lab Immunol, 6(2)216-23, 1999.
Walter Reed Army Institute of Research Report. Comparative Mortality Among US Military Personnel Worldwide During Operations Desert Shield and Desert Storm. January 1995.
Warg-Damiani L, Baldwin CM, Walsh MC, Schwartz ER. Self-reported chemical sensitivity and wartime chemical exposures in Gulf War veterans with and without decreased global health ratings. Mil Med, 163(11)725-6, 1998.
Weddle JR, Chan TC, Thompson K, Paxton H, Kelly DJ, Dasch GA, Strickman D. Effectiveness of a dot-blot immunoassay of anti-Rickettsia tsutsugamushi antibodies for serologic analysis of scrub typhus. Am J Tropical Med & Hygiene, 53(1):43-6, 1995.
Wegman DH, Woods NF, Bailar JC. Invited commentary: how would we know a Gulf War syndrome if we saw one? Am J Epidemiol, 146(9):704-11, 1997.
Weiss B. Neurobehavioral properties of chemical sensitivity syndromes. Neurotoxicol, 19(2):259-68, 1998.
White RF, Diamond R, Krengel M, Lindem K, Feldman RG: Validation of the NES2 in Patients with Neurologic Disorders. Neurotoxicol Teratol, 18:441-8, 1996.
Wickelgren I. Rat model for Gulf War syndrome? Science, 278(5342):1404, 1997.
Wilson BW, Henderson JD, Spencer PS. Clinical effects of low-level exposures to chemical warfare agents in mice and chickens. Drug Chem Toxicol, 21 Suppl, 1:183-90, 1998.
Winter RW, Cornell KA, Johnson LL, Ignatushchenko M, Hinrichs DJ, Riscoe MK. Potentiation of the antimalarial agent rufigallol. Antimicrob Agents Chemother, 40:1408-11, 1996.
Winter RW, Ignatushchenko M, Ogundahunsi OAT, Cornell KA, Johnson LL, Oduola AMJ, Hinrichs DJ, Riscoe MK. A radical mechanism for potentiation of antimalarial oxidant drugs. Antimicrob Agents Chemother, 41:1449-54, 1997.
Wolfe J, Brown P, Kelley J. Reassessing war stress: exposure and the Gulf War. J Soc Issues, 1993.
Wolfe J, Brown PJ, Kelley JM. Reformulating war stress: Exposure and the Persian Gulf War. J Soc Issues, 49, 15-31. 1993.
Wolfe J, Erickson DJ, Sharkansky EJ, King LA, King DW. Course and predictors of PTSD among Gulf War veterans: A prospective analysis. J Consult Clin Psychol, (in press).
Wolfe J, Kelley JM, Buscela ML, Mark WR. Ft. Devens Reunion Survey: Report of Phase I. In R. Rosenheck et al. (eds.), War-zone stress among returning Persian Gulf troops: Final Report. Legislative report to Congress, 1992.
Wolfe J, Proctor S, Davis JD, Sullivan M, Friedman M. Health symptoms reported by Gulf War veterans two years after return. Am J Ind Med, (33)104 -13, 1998.
Wolfe J, Proctor SP, White RF, Friedman M. Letter to the Editor. Am J Epidemiol, 148:402, 1998.
Wolfe J, Proctor SP, White RF, Friedman MJ. Re: Is Gulf War syndrome due to stress: The evidence reexamined. Am J Epidemiol, 148(4):402-3, 1998.
Wolfe J, Sharkansky EJ, Read J, Dawson R, Martin J, Ouimette PC. Sexual harassment and assault as predictors of PTSD symptomatology among U.S. female Persian Gulf War personnel. Journal of Interpersonal Violence, (13)40-57, 1998.
Wolfe J. Preliminary report of reunion survey on Desert Storm returnees. In R. Rosenheck, et al. (eds.), War-zone stress among returning Persian Gulf troops: A preliminary report. Legislative report to Congress, 1991.
Woodward SH, Friedman MJ, Bliwise DL. Sleep and depression in combat-related PTSD inpatients. Biological Psychiatry, (39)182-92, 1996.
Writer JV, DeFraites RF, Brundage JF. Comparative mortality among US military personnel in the Persian Gulf region and worldwide during Operations Desert Shield and Desert Storm. JAMA, 275(2):118-21, 1996.
Yamaguchi K, Near R, Shneider A, Cui H, Ju S-T, Sherr DH. Fluoranthene induced apoptosis in murine T cell hybridomas is independent of the aromatic hydrocarbon receptor. Toxicol Appl Pharmacol, 139:144, 1996.
Yamaguchi K, Near RI, Matulka RA, Shneider A, Toselli P, Trombino AF, Sherr DH. Activation of the aryl hydrocarbon receptor/transcription factor and bone marrow stromal cell-dependent preB cell apoptosis. J Immunol, 158:2165-73, 1997.
Yokoyama K, Araki S, Murata K, Nishkitani M, Okumura T, Ishimatsu S, Yakasu N, White R. Chronic neurobehavioral effects of Tokyo subway sarin poisoning in relation to Posttraumatic Stress Disorder. Arch Environ Health, 53:249-56, 1998.
Zhang Q, Zhou X-D, Denny T, Ottenweller J, Lange G, LaManca J, et al. Changes in immune parameters are seen in Gulf veterans but not civilians with chronic fatigue syndrome. Clin Diagn Lab Immunol, 6:6-13, 1999.
Zhou Y, Cheng YS. Characterization of emissions from kerosene heaters in an unvented tent. Aerosol Sci Technol, (submitted).
Note: Additional resources can be found at the Gulf War Medical Research Library. This on-line library is a collaborative effort of three departments - the Defense Department, the Department of Veterans Affairs, and the Department of Health and Human Services.