Office of Research & Development |
 |
A. Merit Review Award Program
B. Career Development Award Program
C. Special Funding Solicitations
D. Research Career Scientist
E. Other Office of Research and Development (ORD) Services
A. Eligibility Rules
B. Application for Eligibility
A. Prior to Application Submission (e.g. Letters of Intent)
1. Merit Review Applications for Drug Development
2. Collaborative Merit Review Applications
3. BLRD Merit Review Award for Validation of Studies of Importance to Veteran Health (I01)
4. BLRD Interagency Preclinical TBI Resource Center (I50)
5. Permission to Exceed Merit Review Budget Cap
6. Merit Review Applications Using Million Veteran Program (MVP) Data
7. Precision Oncology Actively Managed Portfolio (AMP)
B. BLRD and CSRD Purview for Observational Studies
C. Reporting the Translational Pipeline Stages for Merit Award Applications
D. Electronic Submission of Application
E. Appeals of the Scientific Review of Applications
A. Scientific Review Contacts
B. List of Scientific Review Subcommittees
C. Purview of BLRD and CSRD Scientific Review Groups
D. Scientific Review Success Rates
A. Just-In-Time
B. Start Dates
A. Data Monitoring Committee
B. Progress Reports
C. Modification Requests
D. Final Report
A. Clinical Trials
B. Offsite Research Waivers
C. Participation of Non-Veterans
D. Certificates of Confidentiality
E. Cooperative Research and Development Agreement (CRADA)
F. Biosafety and Laboratory Safety
A. William S. Middleton Award (BLRD)
B. Distinguished Medical Research Scientist (DMRS)
C. John Blair Barnwell Award (CSRD)
Biomedical Laboratory Research and Development (BLRD) and Clinical Science Research and Development (CSRD) services have distinct missions, but share many resources, staff, and processes. Most of the information on this page applies to both BLRD and CSRD.
BLRD supports laboratory and animal research to better understand the causes of and develop or improve treatments for injuries and illnesses that afflict Veterans.
CSRD supports research focusing on intact human beings as the unit of examination. Examples include interventional and effectiveness studies, clinical, epidemiological and technological studies.
If you have questions, see our staff directory, which includes contact information for individuals who manage each of the programs and processes below.
A variety of funding mechanisms are available to BLRD and CSRD investigators. RFAs and Program Announcements are available on the VA Research Intranet (available only from within the VA network). Please refer to the BLRD and CSRD RFA Requirements that describe the budget, eligibility, and LOI requirements for each specific RFA.
Merit Review Award Program: The Merit Review Award Program is an intramural funding mechanism to support investigator-initiated research conducted by eligible VA investigators at VA medical centers or VA-approved sites. This is the principal mechanism for BLRD and CSRD funding of basic, preclinical biomedical and behavioral studies as well as clinical studies of disorders and diseases of importance to the health of Veterans.
The documents listed here are specific to the Merit Review Award Program. Information about eligibility for funding, the application process, scientific review of applications, special circumstances, and management of active research funding may be found below.
Career Development Award Program: The Career Development program is intended to attract, develop, and retain talented researchers working in areas of particular importance to improve the health and care of our nation's Veterans. The Biomedical Laboratory Research & Development Service (BLRD) and the Clinical Science Research & Development Service (CSRD) have a rich history of supporting investigators during their early research careers who have gone on to serve as long-standing, independently funded VA scientists, Center Directors, and research administrators such as ACOS/R, etc. The current Career Development program provides opportunities for clinician and non-clinician biomedical and clinical researchers, including biostatisticians and clinical trialists.
BLRD and CSRD are currently offering the Career Development Award-2 (CDA-2) as a mentored investigator award and Career Development Enhancement Award (CDEA) for senior investigators to obtain additional research training.
The CDA-1 mechanism (similar to early research fellowship) is available for selected priority areas: psychiatry, applicants from MSIs, and Veteran scientists.
C. Special Funding Solicitations: At times, special funding mechanisms are made available. These may be based on portfolio needs, opportunities to work with partner agencies, etc.
D. Research Career Scientist: BLRD and CSRD recognize the critical contributions that non-clinician scientists make to our research program. The intramural nature of VA research encourages our investigators to make VA their primary professional focus. This includes participation in local and national committees, leadership of research core facilities, collaboration with clinician scientists, teaching, mentoring, and supervising junior investigators, and stewardship of shared resources.
In recognition of these efforts, we offer a funding mechanism to support the salaries of non-clinician scientists who have demonstrated extraordinary contributions to VA research beyond their own funded studies and programs: the Research Career Scientist (RCS) award.
The RCS award provides 5-years of salary support. The Senior Research Career Scientist (SRCS) award is provided to highly productive international leaders, who have achieved wide recognition of accomplishments in their research area, and provides 7-years of salary support. For more information about the RCS program, please refer to the documents below.
RCS applications are accepted electronically via Grants.gov. Submission deadlines may be found in Table 3 of the RCS funding opportunity document (RFA). The RCS RFA must be used in conjunction with the VA version of the SF424 (R&R) Application Guide.
Inquiries about the RCS Program should be directed to rcs.materials@va.gov.
1. Office of Research and Development Program Guide 1200.20: Research Career Scientist
2. Template for RCS Mid-Term Reports for BLRD and CSRD Services
3. Format for Curriculum Vitae for Research Career Scientist Applications
4. Tips On Preparing Your Research Career Scientist Application
5. RCS Frequently Asked Questions
A. Eligibility Rules: Unless otherwise specified in a funding opportunity, the following rules govern eligibility for research funding:
BLRD
The Biomedical Laboratory Research and Development (BLRD) Service is temporarily suspending the non-clinician eligibility program starting with the Spring (March) 2024 submission deadline.
Non-clinician investigators who do not currently have BLRD eligibility and are interested in submitting an application to BLRD will be required to submit a letter of intent (LOI) prior to review through the pre-application (I02) process in eRA. An LOI approval memo must be included in a submitted application from a non-clinician investigator. Detailed information on the LOI/pre-application process will follow. For spring 2024, LOIs via the pre-application process will be due November 1, 2023. The LOI/pre-application RFA will be posted on RFAs and Program Announcements page at vaww.research.va.gov/funding/rfa.cfm (Intranet only, copy and paste the URL into your browser if you have intranet access.)
For submission of applications to a BLRD request for applications, the following must be met:
CSRD:
Investigators must have a VA-paid clinical appointment of at least 5/8ths time and dedicated VA research space to conduct their VA-funded research by the time the funding begins.
A. Pre-Applications: All application submissions to Investigators, Scientific Review, and Management (ISRM) require a pre-application (including resubmissions). Details about pre-application requirements and submission deadlines may be found in the Request for Application (RFA) and pre-application RFA for the respective funding mechanism.
These documents are published on the "ISRM Notices of Special Interest (NOSIs) and Requests for Applications (RFAs)" page on the VA Research Intranet at vaww.research.va.gov/funding (VA Intranet only, copy and paste the URL into your browser if you are within the VA network.)
Additional information about pre-application requirements for certain funding mechanisms may be found below:
A Decision Tool has been created to help you determine whether your proposal fits our definition of a clinical trial.
1. Merit Review Applications for Drug Development
The application process for a BLRD Merit Review Award for Lead Isolation and Optimization and Pre-IND studies of Drugs and Biologics BLRDbegins with the preparation and submission of a Letter of Intent (LOI). Please note that a full proposal may not be submitted without an approved LOI. The primary purpose of the LOI is to provide BLRD with the opportunity to determine if proposed studies will jointly address a critically important area of concern that is prevalent in the Veteran population; and whether or not the proposed studies fit within the BLRD purview. In addition, submission of the LOI allows BLRD the opportunity to access and provide feedback on the clinical and/or scientific focus and design of the collaborative merit reviews, the proposed innovations, and the overall impact on the veteran population.
The goal of this funding opportunity is to accelerate translation of research from demonstration of efficacy in vivo to submission of an investigational new drug application (IND) to the FDA. To accomplish this mission, BLRD will provide resources to support pharmacological and toxicological testing or manufacturing services for a lead agent.
In order to be eligible to apply for funding under this opportunity, an investigator must have a validated target and at least one lead agent (or no more than 3 optimized lead agents for a target) with a pending patent. The lead agent must have the potential for commercial licensing and to be developed further for clinical use.
Additional information about the Drug Development LOI may be found here:
2. Collaborative Merit Review Applications
The application process for a BLRD and CSRD Collaborative Merit Review award begins with the preparation and submission of a Letter of Intent (LOI). Please note that a full proposal may not be submitted without an approved LOI. The primary purpose of the LOI is to provide BLRD and CSRD with the opportunity to determine if proposed studies will jointly address a critically important area of concern that is prevalent in the Veteran population; and whether or not the proposed studies fit within the BLRD or CSRD purview. In addition, submission of the LOI allows BLRD and CSRD the opportunity to access and provide feedback on the clinical and/or scientific focus and design of the collaborative merit reviews, the proposed innovations, and the overall impact on the veteran population.
The purpose of this funding opportunity is to invite applications for collaborative I01 projects from multi-disciplinary teams to expand, improve, or transform the understanding of the etiology, pathogenesis, and/or genetics of suicidality, suicide prevention, posttraumatic stress disorder, traumatic brain injury, pain, and opioid addiction. For a collaborative set of collaborative I01s, each site has its own Program Director(s)/Principal Investigators(s), and the program provides a mechanism for cross-site coordination and communication. Collaborative studies are appropriate to address research questions beyond the capacity of a single-site investigation, particularly to accommodate collaborations among sites with diverse expertise, perspectives, and contributions.
At least 3 applications must be submitted as part of the collaborative research application. A shared Overall Research Strategy is required for the linked applications.
Additional information about the Collaborative LOI may be found here:
3. BLRD Merit Review Pilot Awards for Validation of Studies of Importance to Veteran Health (I21)
The application process for a BLRD Validation of Studies of Importance to Veteran Health Merit Review award begins with the preparation and submission of a Letter of Intent (LOI). Please note that a full proposal may not be submitted without an approved LOI. The primary purpose of the LOI is to provide BLRD with the opportunity to determine if proposed studies will jointly address a critically important area of concern that is prevalent in the Veteran population; and whether or not the proposed studies fit within the BLRD purview. In addition, submission of the LOI allows BLRD the opportunity to access and provide feedback on the clinical and/or scientific focus and design of the collaborative merit reviews, the proposed innovations, and the overall impact on the veteran population. The studies to be validated must have important implications in terms of the etiology, pathogenesis, and/or genetics of service related illness and injury in or illnesses which are common in US military Veterans. There are thee RFAs supporting pilot studies to validate novel animal or cell-based models of diseases, clinically relevant findings from population studies, and clinically significant novel therapeutic targets and approaches.
4. BLRD Interagency Preclinical TBI Resource Center (I50)
The application process for the Interagency Preclinical TBI Resource Center begins with the preparation and submission of a Letter of Intent (LOI). Please note that a full proposal may not be submitted without an approved LOI. The primary purpose of the LOI is to provide BLRD with the opportunity to determine if the proposed activities meet the objective of the RFA, to establish a VA-funded interagency center that will work with TBI researchers funded by relevant US federal government agencies to support and accelerate the development of new candidate therapies for TBI. In addition, submission of the LOI allows BLRD the opportunity to access and provide feedback on the clinical and/or scientific focus and design of the merit reviews, the proposed innovations, and the overall impact on the veteran population.
Additional information about the Interagency Preclinical TBI Resource Center LOI may be found here:
5. Permission to Exceed Merit Review Budget Cap
Information about permission to exceed the Merit Review Budget Cap may be found here:
6. Merit Review Applications Using Million Veteran Program (MVP) Data
Investigators submitting Merit Review applications that include access to MVP data are required to register in GENHUB and follow and intent to submit (ITS) process. Information about MVP applications may be found on the ORD intranet site. If you have VA network access, copy and paste the URLs into your browser.
7. Precision Oncology Actively Managed Portfolio (AMP)
The application process to one of the Precision Oncology AMP RFAs begins with the preparation and submission of a Letter of Intent (LOI). Please note that a full proposal may not be submitted without an approved LOI. The primary purpose of the LOI is to provide CSRD with the opportunity to determine if the proposed activities meet the objectives of the Precision Oncology AMP RFAs. There are currently 2 RFAs, one for clinical trials and one for non-clinical trials, which have different LOI requirements and templates.
Additional information about the Precision Oncology AMP LOIs may be found here:
B. BLRD and CSRD Purview for Observational Studies
While an LOI is not required for most observational studies, determining whether an observational study falls under BLRD or CSRD purview can be difficult. The decision tool below is intended to help make that determination. The outcomes being measured or whether the study is prospective or retrospective are not determinative factors when considering BLRD versus CSRD purview. Purview depends on the methods utilized, how the study materials are obtained, and how the researchers interact with patients. Investigators are strongly encouraged to contact their Scientific Program Manager to discuss purview of any observational studies they want to submit to BLRD or CSRD. Applications submitted to RFAs outside of purview may be withdrawn from review.
C. Reporting the Translational Pipeline Stages for Merit Award Applications
To enhance the real-world impact of preclinical research aimed to improve Veterans healthcare, BLRD is piloting a new appendix for Merit Review Award applications that describes the translational stages of their proposed projects. A VA-ORD Translational Pipeline (TP) Stages form has been developed and will be a required attachment all BLRD applications submitted to the Parent RFA (BX-XX-001) starting in Spring 2024.
New BLRD RFA Requirement for Translational Stage Reporting webinar slides
D. Appeals of the Scientific Review of Applications: To ensure the fairness of the Merit Review process, BLRD and CSRD have a mechanism to formally appeal the recommendation of a Merit Review Subcommittee if the Principal Investigator (PI) has evidence of serious flaws in the review of a Merit Review proposal. The process is described in this document: Merit Review Appeal Process.
E. Electronic Submission of Application: Applications for funding are submitted electronically. The process is the same for all ORD services, and can be found here.
The Joint BLRD and CSRD Services Scientific Review Board is the Federal Advisory Committee responsible for the scientific review of BLRD and CSRD funding proposals. The Committee is comprised of subcommittees that serve as the review groups. The Portfolio Manager of each subcommittee is the designated Federal Official in charge of the Panel meeting and is responsible for conducting the meeting in accordance with the policies of VHA, ORD, BLRD, CSRD, and the Federal Advisory Committee Act.
B. List of Scientific Review Subcommittees
C. Purview of BLRD and CSRD Scientific Review Groups
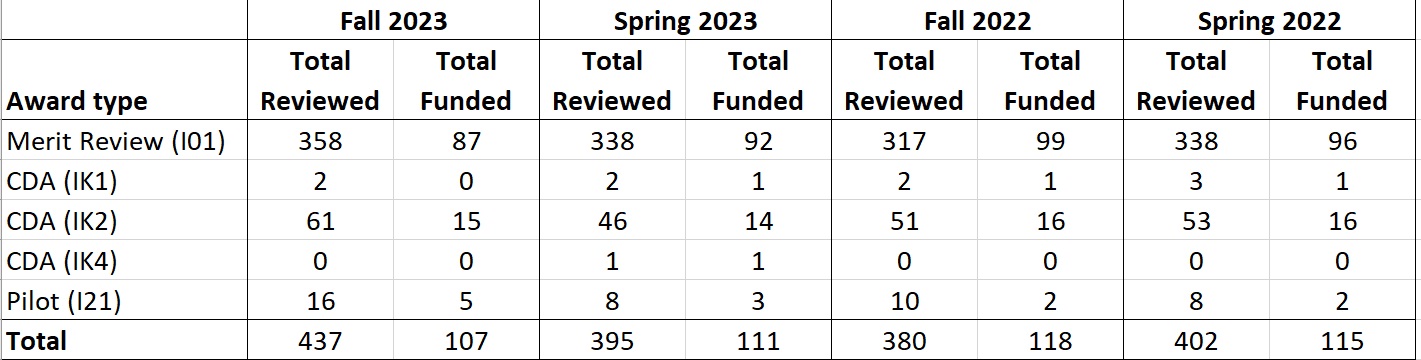
D. Scientific Review Success Rates
Once applications have been selected for funding, we will notify your Research Office and begin the Just-in-Time (JIT) process. This allows investigators and Research Office staff to wait to prepare certain required documents until after the scientific review process.
Details about using JIT may be found here: Just-in-Time (JIT) Forms, Templates, and Instructions
A. Progress Reports: BLRD and CSRD utilize the Research Performance Progress Report (RPPR) module of the NIH Electronic Research Administration (eRA) Commons site. While the specific questions asked may vary based on the funding service and opportunity type, the process is the same for all ORD services, and may be found here: Federal-wide Research Performance Progress Report (RPPR) for VA Investigators.
B. Project Modification Requests
C. Data Monitoring Committee: The CSRD Data Monitoring Committee (DMC) monitors certain CSRD funded studies that include human subjects, and may involve randomization. The DMC provides an ongoing independent evaluation of the progress of studies, including participant accrual and retention, adverse events monitoring, and analysis plan. DMC review is separate and distinct from Institutional Review Board (IRB) review and approval. The DMC will not evaluate the scientific merit, methodology, or overall design, and budgetary constraints of an IRB approved protocol under review, except where changes to the protocol may result in a change in safety level or monitoring. Additional information is available here: CSRD Data Monitoring Committee
D. Final Reports: If your award requires a final report, your research office will be notified.
A. Clinical Trials: Investigators interested in conducting clinical trials may apply for funding through a number of mechanisms, including Career Development and Merit Review. Most clinical trials require submission and approval of a Letter of Intent (LOI) prior to the full proposal submission.
The following documents apply to clinical trials:
a. Merit Review: vhacoblcsrdrev@va.gov
b. Career Development: vhacadereview@va.gov
c. Clinical Trial: clin-review@va.gov
B. Off-site Research Waivers: It is expected that research funded by BLRD or CSRD will be on-site at a VA Medical Center or other VA location. Investigators who wish to conduct all or part of their research activities offsite must request a waiver using the process outlined on the Off-Site Research page in ORD Policies and Guidance Documents.
C. Participation of Non-Veterans: It is expected that all participants enrolled in a BLRD or CSRD funded study be Veterans. Investigators may wish to enroll non-Veterans must request permission to do so, using the memo template below.
D. Certificates of Confidentiality: Although VA does not issue certificates of confidentiality, we have assembled a FAQ to explain when a certificate might be needed and where to apply for one.
In anticipation of budgetary constraints, BLR&D will not be releasing an RFA for ShEEP for FY24. A limited number of LAM requests to replace critical equipment in VA veterinary medical unit may be considered on a case-by-case basis. Please contact Dr. Alex Chiu (alex.chiu@va.gov) or Ms. Sara Clark (sara.clark@va.gov) for information.
The purpose of the ORD Shared Equipment Programs is to review applications for and provide funding of major resource and shared equipment within Department of Veterans Affairs (VA) medical centers to support biomedical research on behalf of investigators associated with all ORD services. All equipment must be housed within VA Medical Centers. ShEEP and LAM equipment may be placed in VA-leased space but a written waiver must be obtained from BLR&D prior to funding. A copy of the fully executed lease must be attached to the waiver request.
Applications for Shared Equipment Evaluation Program (ShEEP) and Shared Equipment Evaluation Program-Imaging Core (ShEEP-IC) must be submitted through grants.gov. Please refer to the RFAs for detailed instructions. The ShEEP and ShEEP-IC RFAs are posted on the VA Research Intranet, accessible only from within the VA firewall. If you are viewing from within the VA network, please clear your browser and paste this URL http://vaww.research.va.gov/funding/rfa.cfm into your browser.
Acceptance of requests for LAM equipment will be announced at the ORD monthly Field Call. Each station may submit only one request per year. The request may consist of multiple items but not exceeding $500,000 in total. Guidance for submitting a LAM request can be accessed below.
Inquiries and requests should be submitted to VACOInfrastructure@va.gov.
A. William S. Middleton Award (BLRD)
B. Distinguished Medical Research Scientist (DMRS)
C. John Blair Barnwell Award (CSRD)
A. Conflict of Interest, Confidentiality and Non Disclosure Rules
C. Purview of BLRD and CSRD Scientific Review Panels
D. ORD Program Guide 1202.01 - Merit Review Award Program
E. VHA Directive 1200.05 - Requirements for the Protection of Human Subjects in Research
G. ORD Program Guide 1200.16 - Off-Site Research
H. VHA Directive 1200.19 - Presentation Of Research Results
I. VHA Directive 1200.08 - Safety of Personnel and Security of Laboratories Involved in VA Research