Office of Research & Development |
 |

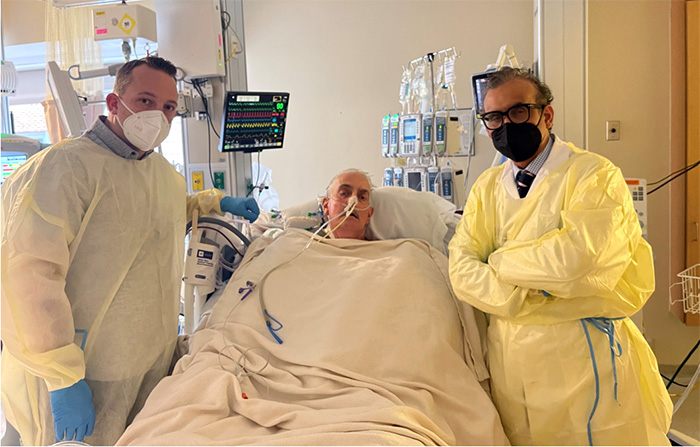
In a groundbreaking operation, David Bennett Sr. (center) received a pig heart transplant in January at the University of Maryland Medical Center, before passing away two months later. Dr. Muhammad Mohiuddin (right), whom former VA scientist Dr. Richard Pierson has collaborated with on studies of transplanting animal organs into humans, was a lead surgeon on the Maryland team. (Photo courtesy of University of Maryland School of Medicine)
March 23, 2022
By Mike Richman
VA Research Communications
"Richard was clearly thinking boldly and creatively about the future of medicinal care for patients whose disease limited the function of a major organ, such as the heart."
Medical research is often a slow, incremental, iterative process. Breakthroughs come about in many cases because of the small contributions of many research groups, each helping to solve critical pieces of the puzzle.
Such was the case in January with a groundbreaking operation at the University of Maryland, where a surgical team transplanted a genetically modified pig heart into a 57-year-old man in a last-ditch effort to save his life. It was the first time a pig’s heart was successfully transplanted into a human being. After not immediately rejecting the heart, a common occurrence in transplantation procedures, the patient lived for two months before passing away on March 8.
Not enough organs to meet the demand
Obstacles remain in normalizing animal-to-human transplantation
This is called xenotransplant technology, which refers to transplanting organs between species—in this case, pig and human.
VA-funded research starting in the 1990s helped uncover knowledge that proved to be key—along with findings by many other groups—to establishing the technique of transplanting a pig’s heart into a person. The researcher leading this work at VA was Dr. Richard Pierson, a specialist in thoracic and cardiac surgery who is now with Massachusetts General Hospital and Harvard University.

VA Study Documents Health Risks for Burn Pit Exposures

VA center training the next generation of researchers in blood clots and inflammation
Could cholesterol medicine reduce dementia risk in seniors?

Million Veteran Program director speaks at international forum
While at the Nashville VA Medical Center in Tennessee from 1994 to 2002, Pierson received funding from VA’s Office of Research and Development to study how to alter the immune system in baboon recipients of genetically modified pig hearts so the heart would not be rejected. This research, he says, was a “proof of principle” intended for eventual application to humans. The pioneering work earned him further funding and recognition with a VA Merit Award in 1998 and a Presidential Early Career Award for Scientists and Engineers (PECASE) in 1999, both of which he used to advance his research.
Pierson was thrilled to hear about the successful transplant at the University of Maryland, where he carried out research on xenotransplantation for about 15 years. Dr. Muhammad Mohiuddin, whom Pierson has collaborated with on studies of transplanting animal organs into humans, was one of the lead surgeons on the Maryland team.
“All of us felt inspired that someone had taken this step, and that it had been successful,” Pierson says. “Many of us would have predicted that a heart with genetic modifications probably would be able to function relatively normally in a human, but that’s a different thing than proving it can actually happen. This transplant was historic.”
That the patient, David Bennett, lived for two months supported by a pig's heart is remarkable, Pierson says, although he’s disappointed that Bennett didn't live for years.
“Those of us who are advocates for this basic technology hope the heart and patient are going to live for a long time every time we do a transplant,” he says. “The longest that non-human primates have been supported with an orthotopic heart transplant has been six or eight months. But those experiments in many instances have been limited by regulatory decisions to stop the experiment, as in the German experience, or by logistical problems with it being expensive and complicated to take care of an animal with a heart transplant. I’m confident that with the right pig, the right treatments, and the right patient, we really could make this work with what we know today.”
Dr. David Klassen, chief medical officer of the United Network for Organ Sharing, says it took years of basic research by many scientists to pull off the surgery on Bennett.
“Despite his death, this transplant remains an important step toward making xenotransplants possible for patients in need of a heart transplant,” Klassen says. “This ought to remind us of the complexity of these approaches and the many challenges that will remain before these therapies can be developed and widely applied. I’m confident a lot will be learned from this transplant even though it was only one transplant.”
 While working more than 20 years ago at the Nashville VA, Dr. Richard Pierson studied a new method of immunosuppression using heart transplants from genetically modified pigs in baboons. The research served as a "proof of principle" for eventual application to humans.
While working more than 20 years ago at the Nashville VA, Dr. Richard Pierson studied a new method of immunosuppression using heart transplants from genetically modified pigs in baboons. The research served as a "proof of principle" for eventual application to humans.
For decades, scientists have experimented with animal organs to make up for the severe shortage of human organs donated for transplant. But transplantation efforts have failed largely because the patients’ bodies quickly rejected the animal organs. In one of the most publicized cases, a baboon heart was placed in a dying infant known as “Baby Fae” in 1984, but the infant lived for only 21 days. It was later learned that her blood type didn’t match that of the baboon.
In terms of size, a pig’s heart is a good candidate for transplantation into a human being. However, pigs express three main sugars that humans make antibodies against. An antibody is a protein used by the immune system to identify and neutralize harmful substances, such as bacteria and viruses. “If you knock out all three of those sugars, the pig and human are much more compatible in relation to any type of organ,” Pierson says.
When working at the Nashville VA as a heart and lung surgeon starting in 1994, Pierson also studied the transplant of animal organs, such as the heart, lung, and kidney, into humans. He and his team proposed to test a new method of immunosuppression using heart transplants from genetically modified pigs in baboons. Having access to some of the first genetically modified pigs made it possible to do that work, Pierson says.
Pierson proposed testing immunosuppression to block inflammation in the baboon caused by complement activation, a barrier that exists in pig-to-human organ and cell transplants. The researchers used pigs with added human genes to test a series of immunosuppressive drugs to block the inflammation.
“Those pigs allowed us to ask a question about whether we could be a little bit more clever and not just heavy-handed about immunosuppression,” Pierson says. “Our proposal described blocking CD154, a key molecule in a baboon’s immune system. We thought that it would be much safer and more effective. It turned out that our prediction was true. We weren’t the ones who were able to first show it because others were able to get a hold of the right reagents before we did. But we were recognized for the idea that this might be possible. That’s what we were rewarded for.”
Similar to Pierson’s research demonstrating the importance of blocking a molecule in the baboon’s immune system, the Maryland surgical team used an experimental antibody drug to block CD40 in David Bennett’s immune system in January. The molecular partner of CD154 is CD140. Blocking either of those molecules interferes with the pathway, Pierson notes.
“We proposed that this novel way of immunosuppression, blocking the CD154/CD40 co-stimulation pathway, would be a big advantage,” Pierson says.
But for the pig-to-human transplant to be successful, several sugar genes in the pig needed to be deleted, and several anti-inflammatory human genes had to be added. Thus, Pierson’s work was just one of many lines of research required to carry out the transplant.
“A pig organ doesn’t fare well in human blood circulation, at least initially, because of certain antibodies, which glom into the surface of the blood vessel lining cells and trigger complement activation,” Pierson says. “Downstream from that, the organ basically turns black and dies rather quickly. By engineering the pig so the enzyme that decorates the cells with that sugar is missing, as it is missing in humans, the incompatibility for that specific sugar goes away.”
In 1998, Pierson received an VA Merit Award that lasted 11 years after several renewals to study how blocking a protein mainly expressed in activated T cells, alone or together with other immunosuppression methods, improves the chances a pig’s heart can be transplanted into a non-human primate like a baboon or monkey. Because the Nashville VA lacked large-animal housing capability, he and his team transplanted hearts from pigs into baboons and monkeys at Vanderbilt.
VA nominated him for a five-year PECASE award, which he received in 1999, to further support the research he conducted through his VA funding.
In 2002, Dr. Bartley Griffith recruited Pierson to the University of Maryland. Griffith, clinical director of the Cardiac Xenotransplantation Program at the University of Maryland School of Medicine (UMSOM), was the surgeon who implanted the pig’s heart into Bennett.
After arriving at Maryland, Pierson helped set up a lab team to work on transplant immunology and assist with the university’s clinical transplant program. In addition to studying heart xenotransplantation, he received funding from the National Institutes of Health (NIH) for research on transplanting lungs from pig to human. He worked with Muhammad Mohiuddin when the latter was at NIH. They showed that blocking the co-stimulation pathway could prolong genetically modified pig hearts up to 900 days.
“Dr. Pierson is a leader in the field of xenotransplantation, and I have appreciated working with him in the past,” says Mohiuddin, scientific director of UMSOM’s Cardiac Xenotransplantation Program. “I consider him a friend and admire his expertise as a xenotransplant researcher. Through our distinct research efforts, we gain invaluable insights from each other, and together we will advance this field toward human clinical trials.”
When Pierson left Maryland in 2017, Mohiuddin took over the lab team. During the surgery on Bennett, he directed the removal of the pig’s heart.
Dr. Don Rubin has long been the associate chief of staff for research and development at the Nashville VA. He and Pierson were colleagues at both the Nashville VA and nearby Vanderbilt University.
“Richard was clearly thinking boldly and creatively about the future of medical care for patients whose disease limited the function of a major organ, such as the heart,” Rubin says. “His VA and PECASE awards were for solving how to transplant a pig organ into a patient that otherwise would die without it. The team he helped establish at the University of Maryland transplanted a modified pig heart into a patient because of this advance.”
Researchers hope the procedure on Bennett will provide a plethora of data on xenotransplantation and confirm whether the combination of genetically modified pig hearts and immunosuppression to block the co-stimulation pathway can together help solve the shortage of organs for transplantation. Pierson believes his work will likely be the basis for an immunosuppression regimen when xenotransplantation matures as a clinical option.
Currently, he’s researching pig organs and cells in his lab at Massachusetts General Hospital in efforts to make heart, kidney, lung, and liver xenotransplantation clinically routine.
“We’re close on all of those fronts,” he says. “It’s nothing but fun to be on the bleeding edge of cool science solving clinically important problems.”
Dr. Richard Pierson is confident that if the technology of xenotransplantation takes off as a clinical option, it would basically solve the shortage of organs needed for transplants. At the same time, regulatory obstacles remain in making the procedure part of the medical mainstream.
The FDA has not approved pig heart transplants. The University of Maryland procedure in January was done under “compassionate use,” otherwise known as the FDA’s expanded-access provision, because the patient, David Bennett, had terminal heart disease and no other options were available.
“The FDA focuses not just on efficacy but on safety,” Pierson says. “In efficacy, we think based on the preclinical primate data that a pig’s heart is quite likely to support the life of a human for weeks, months, conceivably for years. If we could show it consistently for months, the FDA would probably let us go ahead with a standard clinical trial. To get a standard clinical trial approved by the FDA, consistency is important. The Germans have worked out a couple of practical problems and demonstrated that they can consistently support baboons with pig hearts. Those pigs have had just three genetic modifications, and the Germans have had survivors out to three months consistently and six months on occasion.”
The FDA, Pierson notes, wants scientists to also show that the “recipe you’re going to use is safe.”
“Regarding the components of the drug regimen that we developed with co-stimulation pathway blockade, including the CD40 antibody drug the Maryland team used, none of those drugs have been approved for use in human beings. So, everyone has been a little nervous. Will the FDA allow us to use co-stimulation pathway blockade in human patients who are also getting an experimental pig organ?
“The answer the FDA has given is yes. You just need to show them why you need to use a previously unapproved drug, that this isn’t just a matter of convenience. You need to show that the experimental drug is necessary to achieve this positive effect with the pig organ. Several groups have shown that you really do need to use co-stimulation pathway blockade. You can’t just use the same medicines that we conventionally use when a human patient gets a human heart or a human kidney. Those conventional drugs haven’t worked, at least in our work so far with baboons.”
The FDA, Pierson explains, also calls for scientists to use organs from pigs reared in housing facilities where they are not exposed to potential pathogens, bacteria, viruses, and parasites that can be transferred to human recipients. “They have to be really clean pigs,” he says. “The logistics of raising a pig in a clean environment is challenging, which translates into expensive.”
In the University of Maryland procedure, the pig was raised in a pathogen-free facility in Alabama and shipped to Maryland after the surgical team identified the need for “compassionate use” to perform the heart transplant.
Can any human accept a pig’s heart?
“You probably want to pick humans who don’t have pre-formed antibodies against these genetically modified pigs,” he says. “We know that if you do have antibodies against a transplanted organ, that usually causes trouble. So, if you’re going to go ahead with clinical trials, you probably want to make sure you have what’s called a negative crossmatch. But other than that, the blood type of the pigs being brought forward for xenotransplantation are all compatible for the blood type of all humans. They are designed to be, if you will, a universal donor.”
--- Mike Richman
The United Network for Organ Sharing, a nonprofit group that oversees the country’s transplant system, reported that a record 41,355 transplants were performed in 2021, the first time more than 40,000 transplants were done in the United States in a single year. Kidney, liver, and heart were the three most transplanted organs.
Although there has been a record number of deceased donors for 11 years in a row, the supply of available organs isn’t enough to meet the need for those needing transplants. Some 6,000 people died in the U.S. in 2020 while on the transplant waiting list.
The following list from the United Network for Organ Sharing, broken down by organ, shows how many people were on the waiting list for transplants as of the morning of March 9, 2022.
All * 106,171
Kidney 89,991
Liver 11,366
Heart 3,414
Kidney/Pancreas 1,830
Lung 1,065
Pancreas 840
Intestine 201
Heart/Lung 40
All candidates will be less than the sum due to candidates waiting for multiple organs.
VA Research Currents archives || Sign up for VA Research updates