Office of Research & Development |
 |

VA Research Currents archive
April 13, 2016
By Mitch Mirkin
VA Research Communications
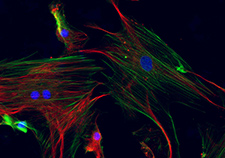
This fluorescence image shows mesenchymal stem cells derived from bone marrow. The blue spots are nuclei, and the green and red filaments are different proteins. (Image by Anup Dutt Sharma, Ames Lab, U.S Dept. of Energy)
A VA and Yale University team, with British and Chinese collaborators, has devised a new way to deliver stem cells for healing diabetic ulcers. They say the method, shown to work in diabetic mice, has a good chance of working in humans.
The experimental treatment uses mesenchymal stem cells, which are found mainly in bone marrow. They naturally turn into various cell types—bone, fat, muscle, cartilage—and in recent years researchers have been trying to learn how to harness them to regenerate tissue.
The VA-Yale team applied the cells to mouse wounds using a collagen scaffold designed to mimic the low-oxygen environment of bone marrow.
The researchers showed that this approach triggers the release of a protein called vascular endothelial growth factor, which spurs the stem cells to grow and differentiate. It also allows the cells to survive longer.
"The mouse model we used is a reasonable model of human wound healing."
Mice that received the cell-infused scaffolds showed improved wound healing, compared with mice that were treated with the collagen scaffolds alone, or with straight injections of the stem cells alone. The mice that received the full treatment showed higher levels of macrophages—a type of white blood cell that eats cellular debris and harmful pathogens. They also had more new fibroblasts—proteins that act as building blocks for the "extracellular matrix" that supports cells—as well as more smooth muscle cells.
The experiment is described in the April 2016 issue of the journal Regenerative Medicine.
To make the scaffold, the team started with a commercially available cell-culture medium containing amino acids, salts, glucose, and vitamins. They added collagen derived from rat tails, along with saline. Then they injected in about two million stem cells.
Next, they put the solution into molds to form a gel, which they then compressed between glass plates to form thin sheets.
They found that when the sheets were rolled up tightly before being applied, they formed a "hypoxic" environment—lacking in oxygen—which, counter-intuitively, has been shown to stimulate stem cell growth.
The scaffolds worked whether they were implanted near the site of the wounds, or applied topically, held on with sutures.
The team, which included Dr. Alan Dardik, a professor of surgery at Yale and chief of vascular surgery at the VA Connecticut Healthcare System, found even greater healing benefits when they added laminin, a naturally occurring protein, to the scaffolds, along with the stem cells.
Diabetes damage nerves in the feet. That means less sensation, so minor cuts or abrasions go unnoticed. Reduced blood flow to the extremities can slow the natural healing process. Without proper attention, these wounds can get infected and worsen to the point of requiring amputation of a foot or leg. Of the 21 million or so Americans with confirmed diabetes—including almost a quarter of the VA patient population—about a quarter will have foot problems as a result. People with diabetes account for about two-thirds of lower-limb amputations in the U.S.
Referring to these health issues, Dardik and his colleagues wrote about their study, "These results suggest that a tissue engineering approach is a viable approach to this difficult and complex clinical problem."
In an email interview, Dardik said there are a couple of reasons why his team believes the therapy will translate well into actual clinical use.
"The mouse model that we used is a reasonable model of human wound healing, and the treatment seems clinically feasible—for example, it worked with topical application," he said. "The barrier will be to determine which cells to use for human therapy, but this work gives us the impetus to continue, as it shows the promise."
The work was supported by VA, Yale, and the National Institutes of Health.