Office of Research & Development |
 |

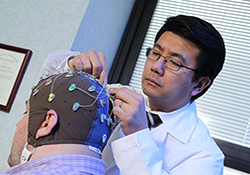
 Drs. Sam Gandy (standing) and Greg Elder at the Bronx VA were part of a team that showed that an experimental drug called BCI-838 could reverse PTSD traits in rats exposed to repetitive blasts, (Photo by Lynne Kantor)
Drs. Sam Gandy (standing) and Greg Elder at the Bronx VA were part of a team that showed that an experimental drug called BCI-838 could reverse PTSD traits in rats exposed to repetitive blasts, (Photo by Lynne Kantor)
June 27, 2019
By Mike Richman
VA Research Communications
"Complex animal models are improving our understanding of why some service members and Veterans have poorer outcomes than others."
About a century ago, soldiers battling in the trenches in World War I were exposed to a variety of blasts at close range, including artillery shells and mortar attacks. The trenches offered some protection from the shrapnel. But those who survived had felt the energy of the blast that penetrated the body and head. Many experienced headaches, memory loss, insomnia, irritability, anxiety, and depression.
Doctors struggled to understand how to best treat this condition. Some described it as “shell shock,”

This image dramatically depicts how blasts—in this case, from the big guns of the USS Iowa in an exercise off the coast of Puerto Rico in 1984—create shock waves in the surrounding water. Scientists are working to better understand the role of such shock waves in traumatic brain injury. (PHAN J. Alan Elliott/USN)
Progress was negligible on this front for decades. But today, it’s a whole new research landscape. Many scientists are focused on understanding the effects on the brain from wounds caused by blasts in the conflicts in Iraq and Afghanistan. Improvised explosive devices have been the weapon of choice for insurgents in the post-9-11 theater, with mild traumatic brain injury (mild TBI), also known as a concussion, evolving as the signature injury. Many of the physical, cognitive, and emotional problems caused by mild TBI are similar to what the service members in World War I and later conflicts experienced.
One way researchers are trying to comprehend this problem is with animal models, namely rats and mice, in laboratory blast simulations or in low-level, open-field explosions. In addition to the dozens of TBI studies it has funded in the past, VA’s Office of Research and Development (ORD) is currently funding about 20 studies aimed at learning how pre-clinical blast exposure impacts the rodents cognitively, physically, and behaviorally. The goal is to apply that information to better understand how blast force effects the human brain.
All but three of the current projects are based on blast-tube simulations. The rest involve open-field exposures to low-level blasts. Those tests have shown that explosions can cause brain injuries to rodents, without the blast waves forcing their head to move like it would in a car accident or contact sport. Scientists believe the pathology of injuries from low-intensity blasts likely differs from that caused by impact injuries.
The VA animal projects are examining the connection, for example, between mild TBI, PTSD, and degenerative brain diseases, such as dementia and chronic traumatic encephalopathy (CTE). Many studies have shown that blast-exposed Veterans are at greater risk for early onset dementia. While TBI is a physical injury to the brain, PTSD is a psychological reaction to a stressful incident that isn’t necessarily associated with a physical injury. Yet, the two co-exist and often involve similar symptoms.
Longitudinal studies—which involve repeated observations of the same study volunteers over long periods of time—are another valuable approach to studying the health impacts of TBI on service members and Veterans. However, Dr. Stuart Hoffman, the scientific lead for TBI in ORD, explains that there are many more animal-based (pre-clinical) than longitudinal human (clinical) studies because of the ability in pre-clinical research to manipulate the exposure to the blast, the number of blasts, and the time after blast—and to conduct tissue and biofluid analyses that can’t be done on humans. These studies also give clinical researchers a good idea where to look in the brains of Veterans and service members for signs of TBI.
Brain's 'error messages' may hold key to PTSD resiliency

Study explores reasons why Veterans seek—or don't seek—PTSD care

A look ahead to the future of rehabilitation
Hoffman is optimistic that the use of rodents will help answer lingering questions about the effects of explosions on the human brain. The basic properties of biological tissue, including the brain, are closely followed across species. Understanding how brain tissue is affected by blasts and how it responds to each successive exposure, as well as how the exposed brain changes over time, is key to understanding the medical implications and therapeutic possibilities, he notes.
“We’ve come a long way and still have a distance to travel,” Hoffman says. “Preclinically, over the last two decades, we’ve moved from open-skull injuries as the primary models, to closed-head and simulated blast injuries, to more recently open-field, low-level blast exposures. We’re also learning about how differences in our genes may affect responses to TBI and subsequent recovery from blast exposure. Complex animal models are improving our understanding of why some service members and Veterans have poorer outcomes than others.”
Two VA programs that do not deal with animal research to study the impact of combat blasts on the human brain, but that complement that work, are the Translational Research Center for TBI and Stress Disorders (TRACTS), based at the VA Boston Healthcare System, with a site at the Michael E. DeBakey VA Medical Center in Houston; and the Chronic Effects of Neurotrauma Consortium (CENC), a joint project of VA and the Department of Defense (DOD).
In the past decade, scientists at TRACTS have been pursuing a longitudinal study. They are investigating the long-term effects of TBI and deployment-related trauma on Veterans who served in Iraq and Afghanistan, and working to develop new treatment approaches for Vets with co-occurring conditions, such as TBI and PTSD. More than 750 Veterans are enrolled in the study.
CENC has a longitudinal study of its own that includes more than 1,600 people out of its total cohort of more than 2,000 Veterans and service members. Each participant was exposed to at least one concussive blast that caused a mild TBI during military service. The goal is to better understand possible chronic and late-life effects of mild TBI, including those that may stem from neurodegeneration.
Ultimately, Hoffman says, these large longitudinal studies will provide definitive knowledge to inform future preclinical modeling and drug development.
Up until 2018, CENC supported a comprehensive basic, or pre-clinical, science program that included a lab-based mouse model. Researchers studied the effects of single and multiple concussive injuries on post-9-11 combat Veterans. The tests showed that mice sustaining repeated concussions over a short period of time—similar to military exposure—and over an extended period of time—similar to contact sports like football—were more likely to develop deposits of tau, a critical protein in the central nervous system.
Abnormal levels of tau have been linked to CTE, which can only be diagnosed posthumously. Its symptoms, which include memory loss, confusion, and irritability, are similar to those of dementia. CTE has been seen in the brains of many deceased football players and other athletes who incurred repeated blows to the head, as well as in deceased Veterans who have been exposed to blasts and other scenarios that can cause head injuries.
No other CENC animal studies are planned at this time, says the consortium’s principal investigator Dr. David Cifu. He’s affiliated with the Hunter Holmes McGuire VA Medical Center in Richmond, Virginia, and Virginia Commonwealth University in Richmond.

Drs. David Cook and Elaine Peskind use a device called a shock tube (shown in foreground) to simulate blasts in rodent models so they can better understand what happens in the brains of soldiers exposed to blasts. (Photo by Christopher Pacheco)
Two proponents of animal research are Dr. David Cook and Dr. Elaine Peskind of the VA Puget Sound Health Care System in Washington state. Cook is a molecular biologist in the Geriatric Research Education and Clinical Center. Peskind is co-director of the Mental Illness Research, Education and Clinical Center.
“Our animal model has given us direction in many ways on what we should look for in the brains of Veterans,” Peskind says. “It is bidirectional. We see something in the animals, we look in the Veterans, and it goes in the other direction, as well. Of course, you can do things in animals that you can’t do in people, such as bluntly sacrificing them and taking their brains out and doing sophisticated analyses.”
Cook and Peskind believe it’s important to experiment with multiple animal models in order to find trends and characteristics that best match those in the human brain.
“There are aspects of a blast exposure that are difficult to simulate in a laboratory,” Cook says. “Still, every way that scientists can experiment with an animal model—either by simulating a blast in a lab or by exposing rodents to a live explosion in an open field—has strengths and weaknesses. The idea of having models to work with where you expose them to a live explosion is good. It’s also good to have laboratory-based means of studying blast exposure in model systems. No one approach is perfect. That’s why it’s important to use multiple approaches.”
Cook explains that some of the lab-based models of blast exposure are better than others. Well-designed shock tubes are among the best, he says, but if the animals are placed in the wrong spot, they will receive sub-optimal exposure to blast waves.
A shock tube is a tunnel-like device in which blast waves are created to replicate an actual explosion. Sensors are positioned in the tube to measure the magnitude of the shock wave and how long it lasts. The tubes rely on compressed gas instead of detonations.
Dr. Greg Elder, a neurologist at the James J. Peter VA Medical Center in the Bronx, New York, also pursues animal testing. He’s interested in the link between repetitive blast exposure and PTSD, knowing that most Veterans who incur mild TBI from combat blasts also experience psychological stressors during demanding assignments in hot climates. He wondered whether animal research could shed light on the PTSD symptoms that these Veterans are facing and point toward a new therapy.
Elder thus began working with Dr. Stephen Ahlers, a neuroscientist at the Naval Medical Research Center in Maryland, to learn if subjecting rats to low-level blasts in a lab can induce PTSD-like traits, without an added psychological stressor. Ahlers had developed an animal model for experiments in which the pressures are comparable to those of a mild TBI.
Dr. Georgina Perez-Garcia, a neuroscientist in Elder’s lab, used that model to show that blasts lead to PTSD-like behaviors in rats, and that prior blast exposure sensitizes animals to react abnormally to a psychological stressor presented many months later. This has led to discussion about a condition called “blast-induced PTSD."
Separately, Elder and Ahlers collaborated with Dr. Sam Gandy of the Bronx VA to test whether a drug could reverse the PTSD traits. The three concluded that an experimental drug (BCI-838) that blocks certain glutamate receptors in the brain reversed many of the PTSD traits in the rats that were exposed to repetitive blasts, including chronic anxiety and altered fear memory. Glutamate receptors are important for neural communication, memory, and learning. BCI-838 has been tested in human clinical trials for depression.
Elder and his colleagues have used a blast tube to carry out tests with rats in a lab. He explains how the blast process works:
“Basically, the anesthetized rat goes in one end. An air pressure generator is at the other end. The pressure wave that is generated is controlled by a set of plastic sheets that break at specified pressures. The pressure wave can vary from low to very high levels. We work at the lower end with pressures that are more comparable to a mild TBI or subclinical blast exposure. This would include what might be experienced in an improvised-explosive-device-related mild TBI, or what military personnel experience in controlled explosions in training exercises.”
Debates on whether the animal should be placed inside or outside the tube, whether the head can be moved by the blast pressure wave, and whether the animal is facing or is perpendicular to the blast have all been investigated extensively, according to ORD’s Hoffman.
Many scientists believe white matter injury spread out over a large area is the main driver behind traumatic brain injury. White matter is found in the deeper tissues of the brain and contains nerve fibers, which are surrounded and insulated by a covering called myelin. Myelin, in part, improves the speed and transmission of electrical nerve signals.
The human brain contains a lot of white matter. There is far less in the rat or mouse brain, in proportion to size. Still, scientists have long used rats as the main model for brain research. Their behavior is more complex, and their brains are larger than those of mice. They are thus easier to study.
Both rats and mice can be altered to have genetic similarities to humans. But brain research has been transitioning toward the mouse because of better imaging equipment and a greater understanding of the mouse’s genetic profile, compared with rats. Plus, the range of genetic and breeding options for making transgenic mice is much larger than it is for rats, according to Cook. In a transgenic procedure, scientists incorporate one or more genes from one organism to another.
Cook and Peskind study mice, which provide what they call a “roadmap” for where to look in Veterans for the pathology of blast-induced injury. The cerebellum and brain stem in a mouse are similar to those in a human brain, and lab research has shown that those two regions are quite vulnerable to the effects of explosions, Peskind says.
For many years, the cerebellum was thought to play an important role only in movement. But emerging evidence has found it also helps control complex cognitive functions. The patterns of white and gray matter in a mouse’s cerebellum are quite similar to those in a human cerebellum, Cook notes.
“There’s no doubt that blast exposure affects many different brain regions,” Cook says, “and the pathologies in those regions combine to yield the complex symptoms of mild TBI. In both human subject studies and in preclinical animal studies, we’re looking carefully at multiple brain regions. Still, we are very interested for several reasons in lower brain regions, such as the cerebellum.”
Cook stresses the need to continually refine animal models so the knowledge gained from these studies is increasingly applicable to the human brain.
“We’re making progress,” Cook says. “But what’s most important is that we’re doing experiments in such a way that when we use an animal, regardless of whether we’re doing it with an actual explosion or in a shock tube, the ultimate standard is the pathology and symptoms in humans. We have to constantly challenge animal models to achieve that. We also have to realize that that’s the big frontier that needs to be addressed. That’s why Elaine and I organized our program the way we did, so we could maximize opportunities to go back and forth between humans and animals, so we can begin to really address this unknown frontier.”
VA Research Currents archives || Sign up for VA Research updates