Office of Research & Development |
 |

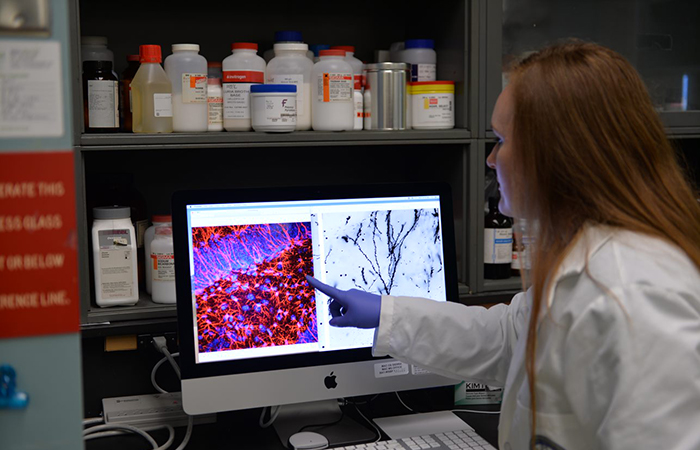
Dr. Whitney Ratliff, who carried out the experiments and analyzed the data in the study, points to injured brain cells in the hippocampus, an important memory center in the brain. (Photo by Timothy J. Westmorland)
October 30, 2020
By Mike Richman
VA Research Communications
"Despite the widespread impact of TBI, we do not have an effective treatment to prevent or reduce long-term damage following an injury. "
A new lab-based study finds that repetitive mild brain trauma causes a moderate loss of connection between nerve cells in an area of the brain that is critical for memory and cognitive performance.
The researchers also detected a major improvement in cell connectivity by using two compounds that have individually been shown to improve the health of brain cells in lab models of neurodegenerative disorders.
The results appeared in the journal Brain Research in July 2020.
Dr. Bruce Citron, a molecular biologist who worked at the Bay Pines VA Healthcare System in Florida at the time, led the study. He is now at the VA New Jersey Health Care System. His postdoctoral fellow, Dr. Whitney Ratliff, carried out the experiments and analyzed the data.
Understanding that traumatic brain injury (TBI) is the signature wound from the post-9-11 conflicts, and that one of the top symptoms of TBI is memory loss, the researchers focused their lab tests on the hippocampus, an important memory center in the brain.
“Despite the widespread impact of TBI, we do not have an effective treatment to prevent or reduce long-term damage following an injury,” says Ratliff, who is now a health research scientist at the Bay Pines VA. “Our goal with this study was to target the biological processes that occur in the brain following injury to improve the health of nerve cells and reduce further injury caused by secondary processes like inflammation.”
The study results complemented a previous behavioral analysis by Ratliff and Citron. They observed memory loss in mice with lab-induced repetitive mild TBIs—even months after the last injury—that signaled major changes in neurons (nerve cells) in the hippocampus. “The shape of the neurons was altered in such a way that would make them less connected and less able to communicate with other neurons,” Ratliff says.
Neurons, the primary units of the brain and nervous system, are responsible for receiving sensory input from the external world, processing and storing information, and transmitting signals through the brain.

Blood pressure drug could prevent posttraumatic headaches

Five ways the Million Veteran Program is transforming our understanding of Veteran health

Traumatic brain injury carries risk for cardiovascular disease in post-9/11 Veterans
Head trauma, PTSD may increase genetic variant's impact on Alzheimer's risk
In the behavioral analysis, the researchers did not observe the same memory deficits when the mice were treated with the two compounds that were later used in the cell connectivity study.
“We are quite surprised by the results from many of our experiments,” Citron says. “Sometimes, the results are exactly the opposite of what we have been hypothesizing. But in this case, the results we saw in the connectivity study were consistent with the clues we obtained in the first set of experiments.”
In the cell connectivity study, the researchers used the two compounds to simultaneously target factors that can influence important signaling pathways in the hippocampus linked to inflammation and antioxidants. The latter protect cells against substances that may play a role in heart disease, cancer, and other health conditions.
One of the compounds (TBHQ) is used as a preservative in foods, based on its antioxidant effects. The other compound (pioglitazone, trade name Actos) is approved by the U.S. Food and Drug Administration (FDA) for treating type 2 diabetes, the dominant form of that illness in adults, and has also shown protective effects in the brain. “Both compounds produce actions that make them good candidates for changing the way the brain responds to injury,” Ratliff says. “By combining them, we hoped we could improve overall outcomes following TBI over just a single treatment.”
Currently, no drugs are FDA-approved for treating traumatic brain injury. However, drugs are used to treat specific TBI symptoms, such as depression, mood swings, and irritability. The Department of Defense and the Defense and Veterans Brain Injury Center estimate that 22 percent of combat casualties from Iraq and Afghanistan involve TBIs, most of which are mild in severity.
Mild TBIs are also called concussions. TBI symptoms also include headaches, sleep disorders, memory lapses, and slower thinking.
To test the effectiveness of the compounds, the researchers divided the mice into four groups: uninjured-untreated, uninjured-treated, injured-untreated, and injured-treated. They put eight mice in each group. The injured mice received five mild impacts to the head, each one a week apart. About 30 minutes after each injury, the researchers injected the mice with the combined treatment or saline as a control. The treatments were calculated and adjusted based on the body weight of the mouse.
“We wanted to increase the impact of the neuroprotective genes while reducing the action of genes that can be detrimental to neurons,” Citron says.
After receiving the impacts to the head, the mice were left undisturbed for eight weeks. At that time, the researchers examined changes in the neurons of the hippocampus.
Only the mice that were injured and untreated, Citron explains, exhibited a loss of nerve cell connectivity in the hippocampus. He and his colleagues measured the lengths, branching, and numbers of the connecting processes extending from each neuron. The injured neurons that received only the saline placebo were reduced in length and less branched, factors that could impact their ability to communicate, he notes.
The research team saw no statistically significant differences between the uninjured mice and those that were injured and treated. “That shows the treatment was beneficial,” Citron says.
According to Ratliff: “When the neurons received the combined treatment, we saw significantly less damage to the neurons. This indicates that our combined treatment provided protection against long-term damage caused by the repeated TBIs.”
Each mouse was anesthetized prior to injury. Knowing that anesthesia can produce changes in the brain, the researchers controlled for anesthesia because of the potential impact on the compounds. “That way, we were able to differentiate changes that occurred because of the injury or treatment, as opposed to changes that may have been caused by the anesthesia itself,” Ratliff notes.
“It has been increasingly recognized that repetitive [brain] injuries are too common among our military personnel,” Ratliff says. “Unfortunately, effective treatments have not yet been developed. A better understanding of the underlying neuropathological mechanisms is necessary to advance treatments that will improve the status of Veterans.”
VA Research Currents archives || Sign up for VA Research updates