Office of Research & Development |
 |
 One out of every three Veterans has osteoarthritis (OA). There are no disease modifying interventions, leaving many suffering for years with pain and disability that compromises quality of life. Regenerative therapies show promise for restoring joint structure and function and reducing pain and disability in our patients, but many challenges to their clinical translation remain.
One out of every three Veterans has osteoarthritis (OA). There are no disease modifying interventions, leaving many suffering for years with pain and disability that compromises quality of life. Regenerative therapies show promise for restoring joint structure and function and reducing pain and disability in our patients, but many challenges to their clinical translation remain.
The Center's mission is to develop cutting-edge strategies for joint tissue regeneration and restoration for Veterans with arthritis and related conditions.
The overall goal of the Center is to restore joint function and transform the long-term health of Veterans with arthritis by:
Dr. Jay Patel (Atlanta; biomaterials, biomechanics, device development), Dr. John O’Donnell (Philadelphia; device translation, regulatory process), & Dr. Nazir Khan (Atlanta; multi-omics characterization, cell-based therapies)
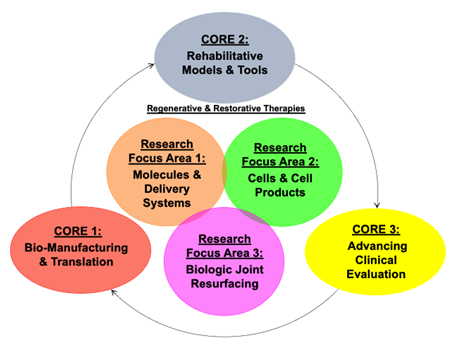
The mission of the Bio-manufacturing & Translation Core (Core 1) is to provide expertise and technician support in the manufacturing and evaluation of therapies from a biofabrication and quality control perspective. We will accomplish this by providing infrastructure and expert guidance required to fabricate and characterize, assure quality and reproducibility, and evaluate safety/tolerability of both cell- and material-based products. These efforts will include high throughput validation, standard procedure documentation, and multi-modal characterization, supporting IND-enabling preclinical studies and clinical translation. As such, the Biomanufacturing & Translation Core will expedite, inform, and ease the fabrication, characterization, and translation of our most promising cell and/or material products to improve Veteran health. The Core will specifically utilize bio-printing facilities at both the Philadelphia and Atlanta sites for construct fabrication and high-throughput models, develop quality control processes including both cell and material characterization, for improved reproducibility, and guide pilot safety-toxicity testing for preparation towards IND-enabling studies.
Dr. Sarah Gullbrand (Philadelphia, structure-function assessment and Philadelphia VA Research Shared Instrument Core Director), Dr. Thomas Schaer (Philadelphia, animal models), Dr. Josh Baxter (Philadelphia, functional outcome measures), & Dr. Greg Myer (Atlanta, functional outcome measures)
The Rehabilitative Models and Tools Core (Core 2) provides expertise in animal model selection, validation and optimization for pre-clinical studies, and in assessing joint structure and function along a continuum of translation from animal models to human patients. These outcome metrics will allow CReATE investigators to objectively measure alterations to joint function with disease and following treatment. The Core provides consultation in study/protocol design, data acquisition and analysis in three main areas: (1) large animal models (2) structure-function imaging (3) clinically relevant functional outcome measures. In area 1, the Core provides expertise in large (minipig, horse) and small (mouse, rat, rabbit) animal model selection, study design and execution for the preclinical evaluation of OA therapeutics. In area 2, the Core can provide protocols for and facilitate access to VA shared equipment for the assessment of joint structure and function – including µCT, MRI, mechanical testing and histology. In area 3, the Core provides access to and consultation on the implementation of markerless motion capture and wearable sensor technology to assess joint function across animal and human studies. The Core is also in the process of installing markerless motion capture infrastructure at both Philadelphia and Atlanta VA sites which can be utilized for human clinical studies.
Dr. Joshua F. Baker (Philadelphia) and Dr. Prathap Jayaram (Atlanta)
The mission of Core 3 is to facilitate the timely, efficient, and effective translation of cutting-edge cartilage and joint regenerative therapies into clinics to improve health and quality of life for Veterans with joint injuries and degenerative joint disease. Core 3 will be the hub for scaling pre-clinical discovery to first-in-human clinical trials, by providing a centralized shared resource to support trial preparedness. Core 3 will provide coordinated and comprehensive researcher-focused support for all stages of clinical research, from study design to closeout. Clinical translation of novel therapies is a challenge and requires an expert team that can facilitate bi-directional bench-to-bedside research and support clinical priorities of the VA. Also necessary is a supportive infrastructure that is prepared for such work. In coordination with Cores 1 and 2, the intellectual resources of Core 3 will facilitate progress of pilot projects and provide regulatory and administrative support for tasks necessary for moving promising therapeutic approaches to the clinic. The specific Aims of Core 3 include 1) pilot and feasibility study planning and data acquisition, 2) Support of first in-human studies, and 3) the facilitation of larger single or multi-site trials. The Core will support CReATE Motion investigators through the provision of staff, regulatory support, as well as seed funding.
These Cores support the three main CReATE Motion Research Focus Areas, centered on Molecules and Delivery Systems (RFA1), Cells and Cell Products (RFA2), and Biologic Joint Resurfacing’ (RFA3). CReATE Motion will fund Pilot Awards and Collaborative Exchanges in each of the 3 research focus areas through a peer-reviewed mechanism. Taken Together, CReATE Motion, with its associated Cores, Trainee, and Pilot Funding, is designed to produce new knowledge, novel products, and advances in clinical care, with a specific focus on functional restoration of the musculoskeletal system.
RFA1 focuses on ‘Molecules and Delivery Systems’. This RFA is developing novel targeted therapeutics based on a deep understanding of joint tissue regulatory networks as well as sustained delivery devices to preserve cartilage and joint health after injury and in early-stage disease. These activities will interface directly with ongoing clinical trials to expedite improved delivery of care to Veterans.
RFA2 focuses on ‘Cells and Cell Products’. This RFA leverages ongoing cell-based clinical trials for OA and expand work in emerging cell-based technologies and cell-products to forward the next generation of cell based regenerative therapeutics to treat mid-stage disease.
RFA3 focuses on ‘Biologic Joint Resurfacing’. This RFA leverages our expertise in biomaterials, biofabrication, and tissue engineered medical products to create living joint implants to resurface damaged joints in late-stage disease.
The combined work of these RFAs is expected to generate a host of new therapeutic targets and strategies to preserve and replace damaged cartilage and translate these to clinical implementation in the Veteran population.

If you are interested in joining the Center, please send your updated Biosketch, Other Support, and a Letter of Endorsement from a Current member to the Center; createmotion@va.gov.