Office of Research & Development |
 |
May 22, 2025
Dr. John Kaplan spoke with Michael W. Hale on the Wreaths Across America Radio Morning Show about the VA Technology Transfer Program. Listen to this podcast.
May 12, 2025
Congratulations to Ms. Renee Shaw, JM, MPH, a Field Technology Transfer Specialist serving VAMCs in Georgia, for completion of her Master of Public Health (MPH) in Public Health Policy & Management from Emory University. Her latest degree will complement her Juris Master from Emory University and her decades worth of benchtop research, as she continues to facilitate intellectual property protection for Veteran healthcare-inspired technologies.
May 8, 2025
Congratulations to Ms. Patricia Cullum, M.Ed., a Regional Technology Transfer Director with the VA Technology Transfer Program (TTP), for being selected as an Elizabeth Dole Foundation Fellow representing New Hampshire. Her selection highlights her dedication to raising awareness and advocating for military and Veteran caregivers.
The Elizabeth Dole Foundation selects Dole Caregiver Fellows from across the nation, including all 50 states, the District of Columbia, and two territories. Selected caregivers represent all eras of war and bring a variety of valuable perspectives to the cohort.
Ms. Cullum's appointment will offer her an immersive multi-year leadership experience, focused on building community across the United States. She will contribute to the picture of what a caregiver looks like, and will offer support to those who self-identify as caregivers. We look forward to her contributions in her new role.
https://www.elizabethdolefoundation.org/campaigns-programs/dole-caregiver-fellows
April 8, 2025
Brandon Johnson, the radiology safety and compliance officer at VA Salt Lake City Health Care System, developed an innovative solution to improve the magnetic resonance imaging (MRI) experience for patients who are deaf or hard-of-hearing. Traditionally, successful MRIs rely on audible commands given by a technologist, but Johnson's innovation seamlessly integrates precise, visual cues into the MRI's bore. This innovation has resulted in faster and more accurate scans, and has left Veterans feeling confident and empowered during the process.
The VA Technology Transfer Program, in conjunction with Johnson, filed for patent protection of this technology. VA TTP licensed the invention to Luminaire, a design company working to fully commercialize the product.
March 17, 2025
The Federal Laboratory Consortium (FLC) published a Labs in Action piece on TrackMate™, developed by Dr. Chetan Jinadatha, MD, MPH (Chief of Infections Disease, Olin E. Teague VA Medical Center). The VA Technology Transfer Program (TTP) licensed TrackMate™ to Xenex Disinfection Services. and it is currently in use at the Olin E. Teague VA Medical Center in Temple, TX.
November 7, 2024
On the Wreaths Across America Radio Morning Show, Michael W. Hale speaks with Dr. John Kaplan, head of the U.S. Department of Veterans Affairs Technology Transfer Program. Dr. Kaplan heads VA's Technology Transfer Program that takes inventions developed on the job by VHA clinicians and researchers and seeks to protect this intellectual property and bring them to market to benefit Veterans, Americans and VA, which receives royalties. Dr. Kaplan also discusses his service in the U.S. Air Force.
September 30, 2024

RAIN Eye Drops
Dr. Julie Schallhorn, an ophthalmologist with the San Francisco VA, has invented a novel assistive device for self-administering eye drops. The dispenser device was designed for patients who find it difficult or painful to manipulate the small, single-use vials that some topical eye-drop medications are stored and administered directly from such as Tafluprost.
Schallhorn's assistive device holds the vial and provides the patients with a larger surface area to squeeze while self-administering their medication. In addition, the dispenser includes a stabilizing guide that the patient places against their face–on the upper part of the cheek just below the eye socket–before squeezing so that they know the vial is well positioned for delivery of the drops and no medicine is inadvertently wasted. The device prototype was refined by the Advanced Platform Technology Center at the Louis Stokes VA Medical Center.
Schallhorn has applied for a patent with the help of VA’s Technology Transfer Program. The intellectual property has now been licensed to RAIN Eye Drops, an online company selling preservative free eye drops. The dispenser device will be included with each new RAIN Eye Drops subscription.
August 5, 2024
Press release from FirstWord PHARMA
July 2, 2024

John Kaplan, PhD, JD
When John Kaplan, PhD, JD, took the helm at the Department of Veteran's Affairs (VA) Tech Transfer Program (TTP), it was his first job in T2. But with his diverse background from the Air Force to patent law and extensive leadership experience, Kaplan transformed the overwhelmed program into a flourishing success story. He shares how he did it, his advice to leaders and his plans for the future of the VA TTP. Listen
May 7, 2024

Anne Burkhart, JD
Ms. Anne Burkhart joined the Department of Veterans Affairs (VA) Technology Transfer Program (TTP) in May 2024 as the Field Technology Transfer Specialist in Ann Arbor, Michigan.
Ms. Anne Burkhart is a registered US Patent Attorney and a member of the Illinois Bar. Before joining the VA, Anne served a number of years in the Technology Transfer Office at University of Illinois Chicago (UIC). Anne worked on hundreds of innovations with UIC faculty from a wide range of colleges including the College of Medicine, College of Dentistry, College of Engineering, College of Nursing and College of Liberal Arts and Sciences. Anne also served several years as internal patent officer and Intellectual Property (IP) attorney for a global medical device company before joining UIC. Anne has extensive IP experience with medical device technologies, software including Artificial Intelligence (AI) and Machine Learning (ML), therapeutics, and other technologies. Ms. Burkhart has served as a speaker at the annual Licensing Executive Society (LES) meeting in 2023, and for the World IP Review (WIPR) Summit in Chicago on a variety of topics including AI and licensing innovations from universities.
Anne has a Bachelor of Science degree in Physics from the University of Cincinnati. Anne attended law school in the evenings while working full-time as a patent agent at a Chicago law firm.
Anne comes from a military family. Her father served in Korea, and her brother is an Army Veteran.
May 6, 2024

Ms. Loraine Kelley
Ms. Loraine Kelley joined the Department of Veterans Affairs Technology Transfer Program (TTP) in May 2024 as a Program Specialist.
Ms. Kelley joined the Marine Corps in June 2001 immediately following high school. After serving in the Marine Corps, Ms. Kelley became an Emergency Medical Technician (EMT) and started teaching at the local community college's EMT department, and teaching CPR and First Aid to the public. In 2012, Ms. Kelley joined the Tucson, Arizona VA Medical Center starting as a phlebotomist. Thereafter, at the Tucson VAMC, Ms. Kelley transitioned to the Cardiology Department and then the Critical Care Department as a program support assistant.
Ms. Kelley enjoys baking, reading, and outdoor activities. Ms. Kelley has a degree in the culinary arts and uses that knowledge to make yummy treats for friends and family members.
Ms. Kelley currently lives in Tucson, Az with her husband, her daughter, and four dogs.
January 31, 2024
The VA Technology Transfer Program (TTP) is thrilled to have received two FLC 2024 awards: 1) Excellence in Technology Transfer for TrackMate: The World's First Disinfection Tracking System, and 2) Technology Transfer Innovation Award for the VA T2 Auditing Program. VA TTP will be recognized alongside fellow 2024 award winners from a range of federal agencies at the FLC Awards Ceremony on April 10th, 2024, sharing the stage with the nation's best in tech transfer. FLC 2024 will be the 50th anniversary of this meeting, highlighting how VA TTP's success will be added to decades of innovative achievements.
January 12, 2024
Marine Corps Spec Ops vet Derek Herrera has built a business around a digital skin inspection tool, known as the "Habit Camera" thanks to a partnership with VA's Technology Transfer Program. Learn more
January 8, 2024
For an article January 2024 issue of Military Officer Magazine, reporter Ms. Hope Seck interviewed Dr. Chetan Jinadatha to discuss the history behind his recently commercialized invention TrackMate, and how the VA Technology Transfer Program (VA TTP) supported its intellectual property protection and market launch. Seck also spoke with VA TTP Director Dr. John Kaplan about the growth of the program since he joined in 2016, highlighting the exponential increase in invention disclosures, royalties, patents, and license agreements. Dr. Rory Cooper, Director of the Human Engineering Research Laboratories (HERL) discussed how his lab has supported VA research and encouraged innovation amongst Veterans. Additionally, Field Technology Transfer Specialist Bhoomija Hariprasad provided commentary on current trends in invention disclosures inspired by the 2022 passage of the PACT Act. Overall, this article captured how VA TTP has gone above and beyond to patent and commercialize VA research.
June 29, 2023
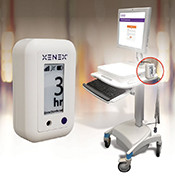
Photo curtesy of Xenex Disinfection Services.
Dr. Chetan Jinadatha of the VA Central Texas Healthcare System invented a device to track the disinfection of portable medical equipment, which will help reduce the spread of infections in hospitals. TrackMate, licensed for production by Xenex Disinfection Services, is a small device that connects to equipment such as IV pumps and vital monitoring machines. It senses moisture from liquid cleaning chemicals or UV light irradiation to keep a log of cleaning, allowing health care workers to quickly see the disinfection history of a piece of equipment before moving it to a new patient’s room. Studies have shown that using TrackMate doubled the amount of equipment cleanings. The device stores data both locally and online, allowing for easier record keeping and compliance monitoring. TrackMate is the world’s first automated disinfection tracking system. (Business Wire, June 26, 2023)
June 1, 2023
The VA Technology Transfer BRAVE Funding Program is an intramural funding mechanism through which eligible VA inventors can receive up to $100,000 to support continued innovation and development of VA owned, or jointly owned, intellectual property assets to better position their technology for commercialization and impact – Bringing Research Advancements for Veterans to Everyone!
Generally, these projects fall into one of the following categories:
July 13, 2022
Photo: ©Getty Images/Mohammed Haneefa Nizamudeen
VA Salt Lake City researchers have created a new drug for treating polycystic kidney disease. The disease is a genetic disorder wherein cysts form in the kidneys. There is presently no cure for the disease. Treatment options are limited and come with undesirable side effects. About half of patients with polycystic kidney disease develop end-stage renal disease, which requires dialysis or kidney transplant. The newly patented VA treatment aims to prevent or at least slow cyst growth in the kidneys using a drug called a platelet aggregation inhibitor, which lowers levels of the hormone arginine vasopressin. This is the second patent issued to VA researchers for the use of a drug called tricagrelor for treating polycystic kidney disease. VA’s Technology Transfer Program is offering a license agreement for drug companies to further develop the drug into an available treatment.
July 13, 2022
Photo: ©iStock/MagicMine
The FDA has granted fast-track review for a drug developed by Central Texas VA Health Care System researchers to treat a rare inflammatory disorder. A research team led by Dr. Sharon DeMorrow created a new galanin receptor inhibitor to treat primary sclerosing cholangitis. The condition is a rare disease that scars bile ducts and can gradually cause serious liver damage. VA’s Technology Transfer Program exclusively licensed the new drug to SteroTherapeutics. The FDA recently granted the new drug Orphan Drug Designation, a status given to new therapies for rare disease that affect fewer than 200,000 people in the United States. The orphan drug status gives the company assistance in the drug development process, tax credits for clinical costs, exemptions from certain FDA fees, and seven years of post-approval marketing exclusivity. The company will next submit an investigational new drug application to the FDA to further develop the drug.
February 28, 2022

The hand-held DropEase device. Photo courtesy of VA Technology Transfer program.
A nurse and case manager at the Cincinnati VA Medical Center’s eye center has invented a new device to make it easier for patients to administer eye drops. Terri Ohlinger noticed that many patients were not using their eye drops, or that they were using too much at once. Many patients with manual dexterity issues have trouble squeezing small eye drop bottles. So with the help of University of Cincinnati engineering students, Ohlinger designed a device called the “DropEase” to make the process easier. The DropEase provides a stable platform for self-administering eye drops, complete with a handle that is easy to squeeze with weak or shaky hands. The device allows users to set a metered dosage to get the proper amount of medication every time. The team designed two versions: a hand-held device and one with an applicator that can be worn like eyeglasses. Ohlinger and her VA colleagues worked with local Veterans to test the prototypes, and received positive feedback.
Ohlinger has applied for a patent with the help of VA’s Technology Transfer Program. Tech Transfer is now marketing the invention to medical device manufacturers for licensing and production.
July 6, 2021

A U.S. Veteran aims an air rifle equipped with a puff trigger. (VA photo)
An assistive device that helps disabled Veterans participate in shooting sports, invented by a VA engineer, has been licensed for commercial manufacture. Seth Hills, an engineer at the Richmond VA Medical Center, created the “puff trigger” device. Hills’ invention allows someone with limited hand or arm function to participate in shooting sports like air rifle competitions by operating the trigger with a puff of air. Ransom International Corp., an Arizona-based company, has negotiated an exclusive patent license agreement with VA to manufacture and commercialize the device. Shooting sports are very popular among Veterans, being featured in such events as the Paralympic Games and the National Veterans Golden Age Games. “Marksmanship is a unique aspect of military service, making shooting sports very popular with Veterans,” said Leif Nelson, director of VA’s Office of National Veterans Sports Programs and Special Events. “Veterans with physical disabilities have an array of shooting sports to choose from through our national rehabilitation events, as well as through their local recreation therapy teams. From air rifle and air pistol to trap shooting and laser rifle, VA has a long history of offering shooting sports programs to our Veterans. We’re excited about this new technology and the potential for more Veterans to have access to these opportunities.” The new device will allow Veterans who may not be able to physically pull a trigger because of a disability to participate in the sports.
June 14, 2021

Dr. BK Kishore
A promising new treatment for obesity has been developed by a VA Salt Lake City scientist. Dr. BK Kishore developed a new way to deliver drugs to act on the P2Y2 purnergic receptor, a protein receptor in the brain involved in both nephrogenic diabetes insipidus and diet-induced obesity. Targeted drug delivery allows medication to be delivered directly to the receptor. The new treatment can “effectively promote burning of excess calories and prevent fat accumulation in a relatively safe manner,” according to Kishore. He has begun a start-up company called ePurines to develop the drug, which signed an exclusive license agreement with VA through the Technology Transfer Program. The agreement allows ePurines to commercialize the purinergic signaling technology developed by Kishore while he worked at VA. VA holds the patent to the new technology. According to the CDC, 42% of adult Americans are clinically obese. About 78% of Veterans have a body mass index above 25. A pharmacological solution to obesity could have a huge impact on the health of both Veterans and the general population.
March 25, 2021

Photo: ©iStock/skynesher
A wheelchair device created by scientists at the Louis Stokes Cleveland VA Medical Center will help prevent injury to Veterans with spinal cord injury. The team developed a sensor that detects the position of a wheelchair user’s feet on the wheelchair footplate. It sends pressure and sensor data to a user’s smartphone in real-time. Patient with spinal cord injury can experience injury when using a wheelchair if they are unaware that their feet are sticking out or dragging on the ground. “Somebody would be operating usually a power wheelchair, although it has happened in manual wheelchairs as well, and their leg or foot might be sticking out from the side of the footrest,” Dr. M. Kristi Henzel, a physical medicine and rehabilitation physician at the Cleveland VAMC, explained to TechLink. “As they would pass through a doorway, for example, it would catch and twist the leg out, leading to injury.” To prevent this, Henzel and her colleagues designed the smart, wireless sensor to warn users foot mispositioning. The device is available for commercial licensing through VA’s Technology Transfer Program.
January 12, 2021

Photo by Megan Kon
A VA-invented mask that could help people communicate more easily during the COVID-19 pandemic is now closer to being available to the public. VA has reached a licensing agreement with Sports Engineering, Inc., for the Clear Talker Mask, invented at the Central Virginia VA Health Care System. The invention is made of clear plastic so that the user’s lips and face are visible. This can improve communication by making it possible to read lips and facial expressions, which cannot be done with the standard surgical masks most often being used during the COVID-19 pandemic. The mask is reusable and is designed to fit most faces. It was created by Seth Hills, Brian Burkhardt, Melissa Oliver, and John Miller at the Central Virginia VA. VA applied for a patent on the invention in September through the Technology Transfer Program. Sports Engineering will develop the product for market.
December 28, 2020
Photo: ©Stock/peterschreiber.media
A new femur prosthetic invented by Dr. Alfred Kuo of the San Francisco VA Medical Center could improve hip replacement surgery and shorten recovery time. Traditional hip replacement surgery often requires a long, painful recovery. The prosthesis designed by Kuo allows for a much shorter segment of the femur bone to be removed than previously required. Conventional hip implants need to be inserted into the femur about five to six inches. But Kuo’s prosthesis only extends into the femur by 90 millimeters, about 3.5 inches. Removing less bone can reduce blood loss during surgery and make the insertion easier for the surgeon. Implantation of the new device also calls for a much smaller incision than for previous prostheses, which makes it easier to redo the surgery if adjustments are needed. VA has filed a patent application for the invention.
August 21, 2020
(Photo by Scott Fuller)
A new mask design invented by Dr. Scott Fuller of the VA Northern California Health Care System could significantly improve safety during procedures of the head and neck. Fuller created a surgical mask with a nasal tube and transparent window for patients to wear. The ongoing COVID-19 pandemic necessitated the creation of the new mask. When performing an endoscopy, in which a fiber-optic camera is inserted into the nose or mouth of a patient, clinicians are at risk of airborne droplets that may contain the virus. Fuller’s invention blocks any droplets or particles from leaving the patient while still allowing the clinician to perform the procedure. He is working with VA’s Human Engineering Research Laboratories to develop prototypes. The Tech Transfer Program has submitted a patent application on Fuller’s behalf and is working to identify companies that can produce the masks for widespread use.
July 17, 2020

Stephanie Nogan Bailey displays the self-leveling walker her team developed. (VA photo)
An adjustable walker that will help users travel over uneven surfaces is one step closer to reaching people who need it. The Self-Leveling Walker was developed by the Advanced Platform Technology Center at the Louis Stokes VA Medical Center and Case Western Reserve University. On level ground, it works like a regular walker. But the user can press a button to shorten the front legs and lengthen the back legs when going up a slope or steps—or vice versa when going down. The walker uses a hydraulic system built into its legs. VA and the university, which co-own the technology, have licensed it to the start-up company LevelMed Technologies to bring the walker to market. “Because there’s no safe way to use standard walkers on stairs or inclines,” explains project manager Stephanie Nogan Bailey, “we saw an unmet need for a walker that can be adjusted at any time.” The hope is that this device will give back freedom of mobility to people with injuries or movement difficulties.
May 1, 2020
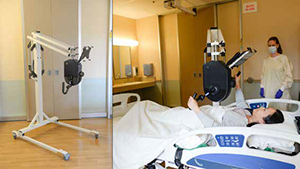
An upper-limb exercise device invented by VA researchers will soon become available to patients and health care systems. The Multi-Purpose Arm Cycle Ergometer for Rehabilitation (M-PACE) allows patients with conditions such as spinal cord injury to participate in a wider range of exercise and rehab activities. Action Manufacturing Inc. and VA have reached an exclusive patent license agreement on the device. The M-PACE was created by researchers at VA’s Minneapolis Adaptive Design and Engineering Program. Prolonged bed rest due to medical treatments or conditions can lead to muscle loss and other problems. With the M-PACE, patients can exercise while lying in bed. It can also be used from a wheelchair or while standing. The device features pedals that can be used with the arms, along with several other configurations. “The M-PACE’s ability to deploy over a bed is particularly important,” explained Dr. Gary Goldish, one of the inventors, to TechLink, “because prolonged bedrest without exercise can lead to rapid deconditioning and can negatively affect many systems of the human body, including the central nervous system, digestive system, and endocrine system.”
March 31, 2020
Photo: ©Stock/©iStock/D-Keine
A VA research team led by Dr. Gianfranco Alpinini of the Richard L. Roudebush Indianapolis VA Medical Center created a new treatment for liver disease. Using mouse studies, the researchers found a compound that improves liver damage linked to several forms of liver disease. The drug could be used as a treatment for liver diseases such as primary sclerosing cholangitis, biliary atresia, and non-alcoholic fatty liver disease. Fatty liver disease is the most common type of liver disease in the Western world. VA has filed a patent for the treatment. Through Tech Transfer agreements, private businesses can now obtain rights to develop this research into life-saving medicines.
March 24, 2020
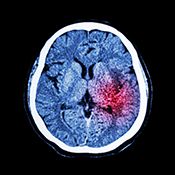
Photo: ©Stock/stockdevil
A new drug developed by VA and international partners could reduce deadly brain damage from stroke. The drug, ZT-1a, has proved effective at reducing brain swelling after stroke in animal models. It targets a pathway in the brain that controls how ions and water move in and out of brain cells. After stroke, malfunctioning proteins can allow too much water into the brain and cause dangerous swelling. ZT-1a stops these proteins from activating. Currently, invasive brain surgery is needed to alleviate this swelling. The drug was developed by an international consortium, including Dr. Dandan Sun of the VA Pittsburgh Health Care System. The consortium was made up of inventors from Xiamen University in China, Exeter University in the United Kingdom, and the VA and University of Pittsburgh. VA has filed a patent for the drug’s use as a stroke treatment.
March 12, 2020

An experimental portable blood-clotting sensor called ClotChip, co-owned by VA and Case Western Reserve University and licensed to the private company XaTek, now has FDA “Breakthrough Device” status. The designation speeds the development of technologies that outperform current products in treating or diagnosing serious conditions. Investigators with VA’s Advanced Platform Technology Center and CWRU developed and tested the technology. It can assess a patient’s clotting ability—based on a single drop of blood—in about 15 minutes, compared with a day or longer for existing methods. “ClotChip is designed to minimize the time and effort for blood-sample preparation. [It can] be used at the doctor’s office or other points of care for patients on anticoagulation therapy [or] antiplatelet therapy, or who have suffered a traumatic injury causing bleeding,” said Dr. Pedram Mohseni in a 2016 CWRU article.
February 25, 2020
(Photo courtesy of CDC)
VA and the University of Maryland are joint owners of a newly issued patent (US 10,537,609 B2) for a peptide-based treatment for Pseudomonas aeruginosa infections. P. aeruginosa can cause infections in the blood, lungs, or other parts of the body after surgery. At highest risk are hospital patients on breathing machines, those with catheters, and those with wounds from surgery or burns. The new potential treatment has been tested successfully in mice and is pending further study. The approach is promising because it uses a specific peptide, or a piece of a protein, to defeat the bacteria and does not rely on antibiotics. P. aeruginosa is increasingly resistant to antibiotics. In 2017, multidrug-resistant P. aeruginosa caused an estimated 32,6000 infections and 2,700 deaths in the U.S. Visit VA’s partner TechLink to read more about this and other available technologies based on VA inventions and discoveries.