Office of Research & Development |
 |
Office of Research & Development |
 |
VA is committed to supporting the research that is needed to improve medical care for Veterans. There is far more research that needs to be done than VA can support, so VA selects the work that is most likely to be valuable. Of all the research that is proposed, typically about 20% gets selected by scientific subject matter experts as scientifically valid and of highest priority for funding. Of that, more than half is research that is best done with human subjects, computer models, analysis of existing data, or collection of data from biological systems other than vertebrate animals. Of the remaining less than half that depends on work with living non-human vertebrate animals, over 99% is done with rats and mice. Less than 5% of that last 1% of the research that depends on living vertebrate animals involves dogs, cats, or nonhuman primates, as shown in the figure below:
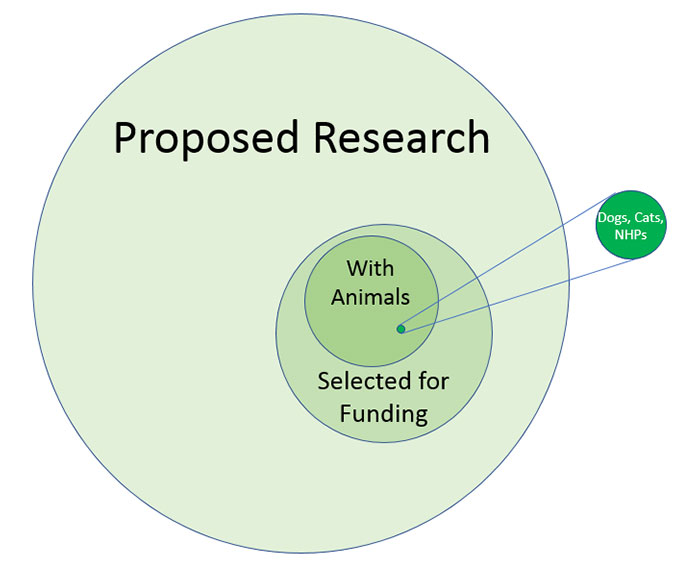
All VA research with animals is subject to careful review and oversight, but research with these three species is scrutinized especially closely, according to procedures that were formally described in Guidance Document AR 2017-001, which was most recently updated July 16, 2020.
Only the protocols for VA research with these species that are shown here are currently approved for work to proceed. For each protocol, there is a link to the following supporting documents:
|
Protocol |
Funding Source |
VA Location |
|
1. Mechanistic Insight of Premature Ventricular Contractions- induced Cardiomyopathy Protocol Form, Feedback Document, and Summary of the Literature |
NIH |
Richmond, VA |
|
Purpose of Research: Premature Ventricular Contractions (PVCs) interfere with proper beating of the heart. This is research into the cellular mechanisms involved, which we need to understand in order to develop better ways to manage PVCs. Reason for Working with Canines: The size of the heart, the rate at which it beats, and the electrical system that coordinates it, are all crucial to making it beat properly. The canine model is used because the canine heart is more similar to those of humans in all of these ways than the hearts of rodents, rabbits, pigs, or sheep are. |
||
|
2. Autonomic Nerve Activity and Cardiac Arrhythmias Protocol Form, Feedback Document, and Summary of the Literature |
American Heart Association |
Richmond, VA |
|
Purpose of Research: Premature ventricular contractions (PVCs) interfere with nerve signals to the heart and can damage heart muscle. This is research into how the loss of nerve signals might be responsible for the damage. Understanding this better is critical to developing better ways to protect the heart. Reasons for Working with Canines: The size of the heart, the rate at which it beats, and the electrical system that coordinates it, are all crucial to making it beat properly. The canine model is used because the canine heart is more similar to those of humans in all of these ways than the hearts of rodents, rabbits, pigs, or sheep are. |
||
|
3. Administration of Intratumoral Immunocytokine to Activate Immune Rejection of Spontaneous Canine Melanoma Protocol Form, Feedback Document, and Summary of the Literature VA funds UW-Madison School of Veterinary Medicine cancer study in dogs (nbc15.com) |
VA |
Madison, WI |
|
Purpose of Research: Service-related to exposure to sun places Veterans at increased risk of melanoma, compared to the general US population. This aggressive skin cancer frequently spreads to other parts of the body, after which it is usually fatal. In dogs, melanoma is a common oral cancer. This research is to evaluate the safety and effects of some new ways of treating melanoma in privately owned pet dogs that have developed the disease spontaneously, and have been brought to a university school of veterinary medicine for care, where the owners choose to have their dogs participate. Reasons for Working with Canines: Because of the similarity of the oral melanoma that develops spontaneously in dogs to the melanoma that humans get, this is an important step in the process of preparing the new treatments for human clinical trials. Both pets and humans benefit from this research. Dogs may hold key to treating cancer in humans (November 27, 2022) |
||
Approved VA Research with Sensitive Species that has been Completed
Approved VA Research with Sensitive Species that has been Completed
|
Protocol |
Funding Source(s) |
Station |
|
1. Stem Cell Therapy for Treatment of Spinal Cord Injury (fully approved before the policy of expanded secondary review of protocols for work with non-human primates was established May 3, 2018) |
VA |
San Diego |
|
Purpose of Research: Over 40,000 US veterans are living with spinal cord injuries (SCI), and there are still no therapies to repair the spinal cord. This research is to explore the possibility that neural stem cells can be used to help bridge the damaged tissue and restore communication across the site of the injury. This is an effort to go beyond helping those with SCI to live with them, the goal is to actually cure the problem. Reasons for Working with Non-Human Primates: It is essential to work with non-human primates because previous work with stem cell transplantation revealed key differences in how the stem cells behaved in non-human primates compared to in rodents; furthermore, the fine motor control of the hand, which is of particular interest in this research, is unique to primate species. |
||
|
2. Contusion Injury as a Model for Spinal Cord Injury (fully approved before the policy of expanded secondary review of protocols for work with non-human primates was established May 3, 2018) |
VA |
San Diego |
|
Purpose of Research: Over 40,000 US veterans are living with spinal cord injuries (SCI), the most common of which are contusion (bruising) injuries, which have important anatomical and functional differences from injuries in which the spinal cord is cut. This research is to gain greater understanding of contusion SCI, and its time course and functional effects, to improve prediction of the outcomes of injuries that people experience, and then be better able to detect the effects of therapies that are applied. Reasons for Working with Non-Human Primates: The anatomical organization and functional roles of the neural pathways from the brain and brainstem to the muscles they control are likely to be important to the time course and pattern of recovery, and non-human primates are much more similar to humans in these ways than rats are, so the outcomes of these studies in non-human primates are much more likely to be meaningful for humans than studies with rats would be. |
||
Approved VA Research with Sensitive Species that has been Completed
VA continues to be committed to supporting the research that is needed to improve medical care for Veterans, and recognizes that a very small portion of that research currently still depends on work with sensitive species of animals. VA is also committed to continuing to reduce the need for sensitive species of animals to be involved in VA research. There are various strategies for doing this, and VA is investing in a multipronged approach, as described in VA's Five-Year Plan for Reducing Research with Sensitive Species, submitted to Congress in December, 2020, as required by PL 116-94, § 249(e).